making salts
1/3
Earn XP
Description and Tags
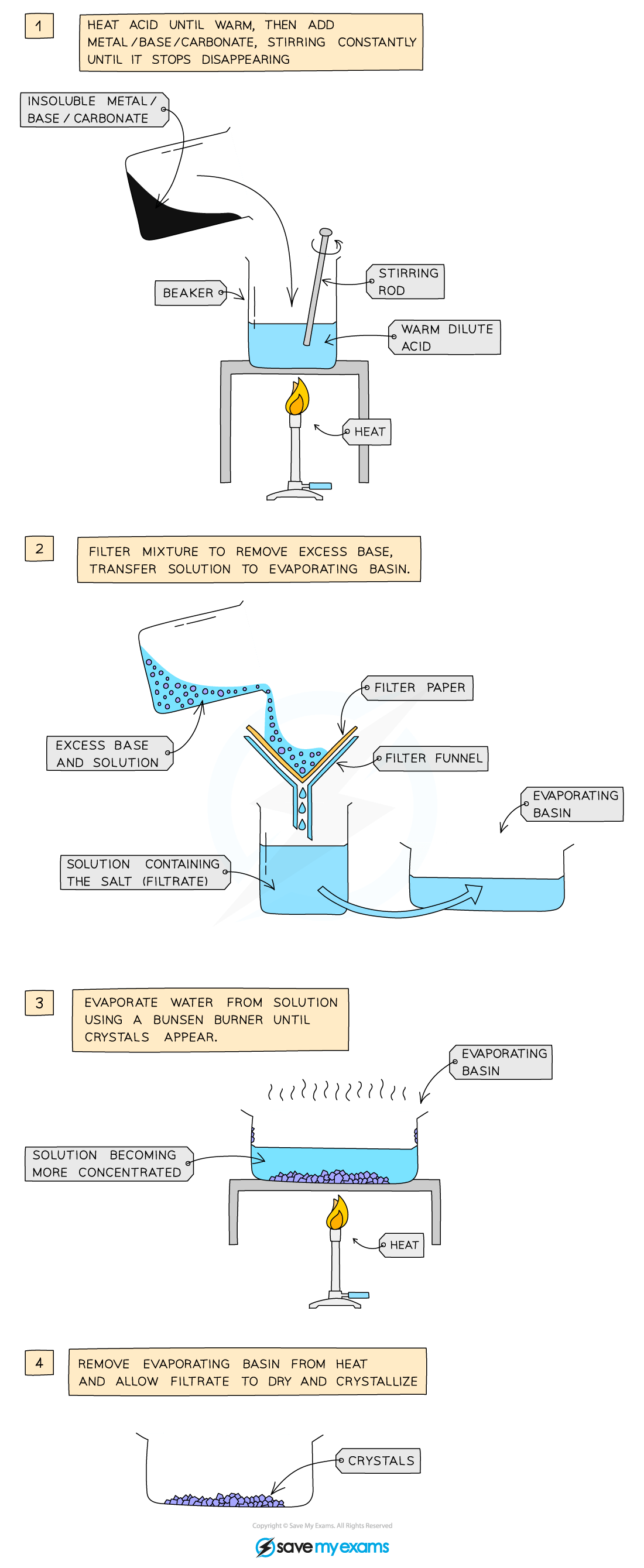
Acids react with metals, bases and carbonates to produce salts. In this practical, you will react sulfuric acid with insoluble copper (II) oxide to prepare an aqueous solution of the salt copper sulfate. You will separate unreacted copper (II) oxide by filtration and follow steps to evaporate the solvent to obtain a pure dry sample of copper sulfate crystals.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
aim
prepare a pure, dry sample of a soluble salt from an insoluble oxide or carbonate
equipment
copper oxide base
sulfuric acid
steps
take a measured amount e.g. 40cm³ of acid using measuring cylinder
pour into beaker
gently heat using a tripod, gauze and bunsen burner for a short period of time
remove beaker from heat and add excess copper oxide
once copper oxide is in excess, must remove this from copper sulfate so crystals are pure - filter using filter paper and funnel, pour beaker contents into paper
pure, blue copper sulfate solution passes through to conical flask
evaporate the water using an evaporating basin
heat evaporating basin - place it on a beaker of boiling water on the tripod above a bunsen burner
as crystals begin to form, stop heating and leave crytals to cool so more form
scrape crystals out, leave to dry in warm oven/dab with filter paper
how do you know when copper oxide is in excess?
no more colour changes (here, mixture is blue when not in excess and black in excess as there is black unreacted copper oxide present)
no more bubbling/effervescence occurs