Midterm 3 Mechanisms NIRRS
5.0(1)
5.0(1)
Card Sorting
1/26
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
1
New cards
Alkene + HX (Cl, Br, I) -> Haloalkene
Intermediate: Carbocation (rearrangement possible)
Regiochemistry: Markovnikov (X)
Stereochemistry: Mixed
Regiochemistry: Markovnikov (X)
Stereochemistry: Mixed
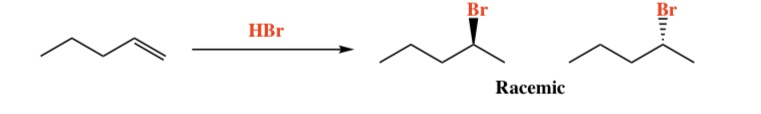
2
New cards
Alkene + H20 (catalyzed by H2SO4) -> Alcohol
Intermediate: Carbocation (rearrangement possible)
Regiochemistry: Markovnikov (OH)
Stereochemistry: Mixed
Regiochemistry: Markovnikov (OH)
Stereochemistry: Mixed
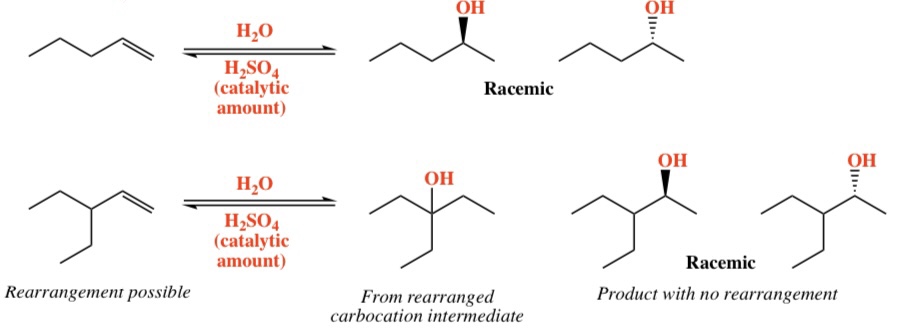
3
New cards
Alkene + X2 (Br, Cl, I) -> Vicinal Dihaloalkanes
Intermediate: 3-membered ring halonium ion
Regiochemistry: N/A
Stereochemistry: Anti
Regiochemistry: N/A
Stereochemistry: Anti
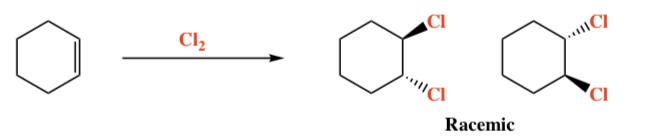
4
New cards
Alkene + X2/H20 -> Halohydrin
Intermediate: 3-membered ring halonium ion
Regiochemistry: Markovnikov (OH)
Stereochemistry: Anti
Regiochemistry: Markovnikov (OH)
Stereochemistry: Anti
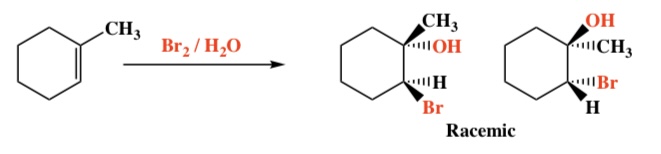
5
New cards
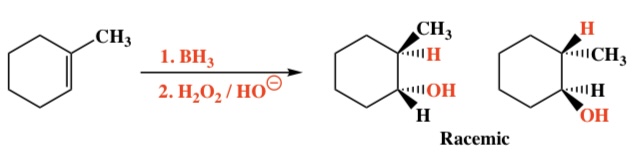
Alkene + BH3, H2O2/HO- ->Alcohol
Intermediate: N/A; 4-membered ring transition state
Regiochemistry: Non-Markovnikov (H on more substituted)
Stereochemistry: Syn
Regiochemistry: Non-Markovnikov (H on more substituted)
Stereochemistry: Syn
6
New cards
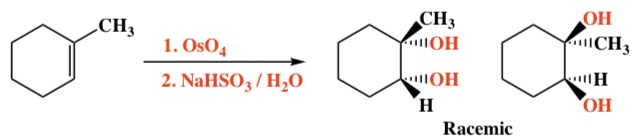
Alkene + OsO4, NAHSO3/H2O -> Vicinal Diols
Intermediate: Cyclic osmate ester
Regiochemistry: N/A
Stereochemistry: Syn
Regiochemistry: N/A
Stereochemistry: Syn
7
New cards
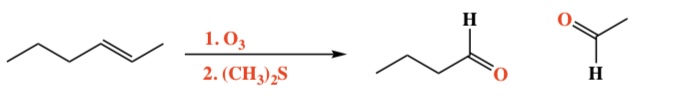
Alkene + O3, (CH3)2S -> Aldehyde + Ketone
Intermediate: Malozonide & Ozonide (C-C bond breaks)
Regiochemistry: N/A
Stereochemistry: N/A
Regiochemistry: N/A
Stereochemistry: N/A
8
New cards
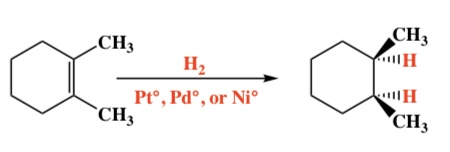
Alkene + H2 (on Pt, Pd, or Ni) -> Alkane
Intermediate: Alkene bonds to metal surface
Regiochemistry: N/A
Stereochemistry: Syn
Regiochemistry: N/A
Stereochemistry: Syn
9
New cards
Alkane + Br2, hv -> Haloalkane
Intermediate: Radical chain process
Regiochemistry: Br ends up on more substituted C atom
Stereochemistry: N/A
Regiochemistry: Br ends up on more substituted C atom
Stereochemistry: N/A
10
New cards
Alkene + ROOR, hv, HBr -> Haloalkane
Intermediate: Radical
Regiochemistry: Non-markovnikov
Stereochemistry: Mixed
Regiochemistry: Non-markovnikov
Stereochemistry: Mixed
11
New cards
Haloalkane + Strong base -> Alkene
Intermediate: N/A; anti-periplanar position; E2
Regiochemistry: Zaitsev
Stereochemistry: Determined by anti-periplanar transition state requirement
Regiochemistry: Zaitsev
Stereochemistry: Determined by anti-periplanar transition state requirement
12
New cards
Vicinal Dihaloalkane + 2NaNH2 -> Internal Alkyne
Intermediate: N/A; Double E2 reaction
Regiochemistry: N/A
Stereochemistry: N/A
Regiochemistry: N/A
Stereochemistry: N/A
13
New cards
Vicinal Dihaloalkane + 3NaNH2, HCl/H2O -> Terminal Alkyne
Intermediate: N/A; Double E2
Regiochemistry: N/A
Stereochemistry: N/A
Regiochemistry: N/A
Stereochemistry: N/A
14
New cards
Internal Alkyne + NaNH2, RCH2X -> Terminal Alkyne
Intermediate: N/A; SN2 reaction; makes C-C bond
Regiochemistry: N/A
Stereochemistry: N/A
Regiochemistry: N/A
Stereochemistry: N/A
15
New cards
Alkyne + Na/NH3 -> Alkene
Intermediate: Radical
Regiochemistry: N/A
Stereochemistry: Anti, E products
Regiochemistry: N/A
Stereochemistry: Anti, E products
16
New cards
Alkyne + H2, Lindlar’s Catalyst -> Alkene
Intermediate: Alkene and H2 adsorb on metal surface
Regiochemistry: N/A
Stereochemistry: Syn, Z products
Regiochemistry: N/A
Stereochemistry: Syn, Z products
17
New cards
Alkyne + H2, Pt/Pd/Ni -> Alkane
Intermediate: Adsorption on metal surface
Regiochemistry: N/A
Stereochemistry: N/A
Regiochemistry: N/A
Stereochemistry: N/A
18
New cards
Alkyne + 2HX (Br, Cl, I) -> Geminal Dihaloalkane
Intermediate: HX reacts with both pi bonds
Regiochemistry: Markovnikov
Stereochemistry: N/A
Regiochemistry: Markovnikov
Stereochemistry: N/A
19
New cards
Alkyne + 2X2 (Br, Cl, I) -> Vicinal Tetrahaloalkane
Intermediate: X2 reacts with both pi bonds
Regiochemistry: N/A
Stereochemistry: N/A
Regiochemistry: N/A
Stereochemistry: N/A
20
New cards
Alkyne + (sia)2BH, H2O2/HO- -> Aldehyde/Ketone
Intermediate: N/A; 4-membered ring transition state
Regiochemistry: Non-markovnikov
Stereochemistry: N/A
Regiochemistry: Non-markovnikov
Stereochemistry: N/A
21
New cards
Alkyne + HgSO4, H2SO4, H2O -> Aldehyde/Ketone
Intermediate: Enol
Regiochemistry: Markovnikov
Stereochemistry: Mixed
Regiochemistry: Markovnikov
Stereochemistry: Mixed
22
New cards
Primary Alcohol -> Alkene
Intermediate: Leaving group; E2 reaction
Regiochemistry: Zaitsev
Stereochemistry: N/A
Regiochemistry: Zaitsev
Stereochemistry: N/A
23
New cards
Primary Alcohol + HX -> Haloalkane
Intermediate: N/A; SN2 reaction
Regiochemistry: N/A
Stereochemistry: Inversion
Regiochemistry: N/A
Stereochemistry: Inversion
24
New cards
Haloalkane + Nucleophile -> SN2 Products
Intermediate: Nucleophile attacks backside of C-leaving group bond
Regiochemistry: N/A
Stereochemistry: Inversion
Regiochemistry: N/A
Stereochemistry: Inversion
25
New cards
Alkene + NBS, hv -> Haloalkene
Intermediate: Allylic radical
Regiochemistry: Most stable alkene product
Stereochemistry: N/A
Regiochemistry: Most stable alkene product
Stereochemistry: N/A
26
New cards
Secondary/Tertiary Alcohol -> Alkene
Intermediate: Leaving group; E1 reaction
Regiochemistry: Zaitsev
Stereochemistry: N/A
Regiochemistry: Zaitsev
Stereochemistry: N/A
27
New cards
Secondary/Tertiary Alcohol + HX -> Haloalkane
Intermediate: N/A; SN1 reaction
Regiochemistry: N/A
Stereochemistry: Scrambled
Regiochemistry: N/A
Stereochemistry: Scrambled