Covalent bonding HL
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
Why are oxygen and ozone dissociated by different wavelengths?
the bonds in ozone have intermediate properties between singly and doubly bonded oxygen. This concept of resonance hybrids can be used to explain the intermediate bond strengths described previously: the bonds are stronger than a single bond but weaker than a double bond. This is why ozone molecules require a longer wavelength of light to break their bonds than the O=O double bond in oxygen molecules, but a shorter wavelength than what would be needed for an oxygen–oxygen single bond.
Delocalisation
molecule is no longer fixed to one bonding position: it is spread over two positions.
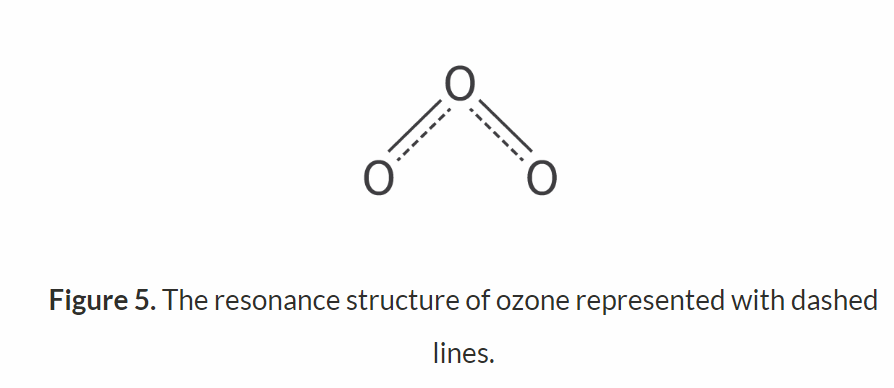
What’s bond order?
A collective term used to describe the properties of single, double and triple bonds, includes bond enthalpy and bond length.
Single bonds have a bond order of 1, double bonds have a bond order of 2 and the bond order of a triple bond is 3.
What is resonance structure? and how to identify it
It has a bond order of 1.5 (it has to be between 1 to 2, if it isn’t add a 1 Infront of the fraction.
What is benze?
C6H6 (cyclic hexagonal structure)
Each carbon has a delocalised eletron
Wy does benzene not behave like a molecules with single and double bonds?
Physical evidence:
Carbon to carbon bond lengths and strengths are equal;
Benzene is a planar molecule;
C–C–C bond angles are equal
Chemical evidence:
Enthalpy of hydrogenation is less than predicted;
Benzene undergoes substitution reactions, not addition reactions;
Only one isomer exists for the reaction of benzene with chlorine or bromine.
What elements can form expanded octets ?
Phosphorus, Sulfur and chlorine
Resonance energy
The additional stability that a resonance hybrid molecule has due to the delocalised electrons. Also known as stabilisation energy.
Why is benzene not reactive/stable/dont take part in reactions?
This is because it would involve first disrupting the entire cloud of delocalised electrons which requires a lot of energy. The resonance energy would have to be supplied and the product would be less stable without the delocalised ring of electrons.
Instead, benzene undergoes a type of reaction usually only associated with molecules with single bonds, which involves the replacement of atoms or groups of atoms with a part of the reactant molecule.
Substitution reaction takes place within a benzene but addition and hydrogenation do not.
Draw all the different structures of benzene
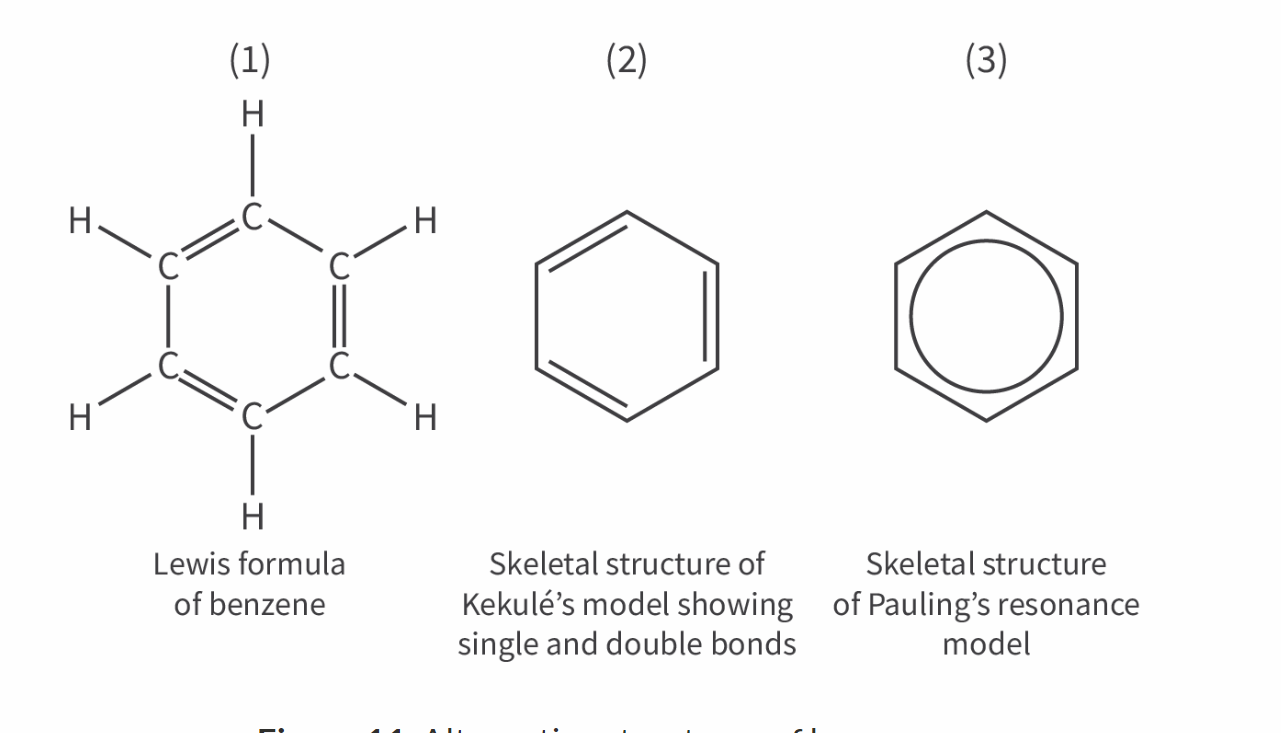
Reasons for stability of octets
Achieving stability with an incomplete octet is typically only possible for smaller atoms and can be linked to their position on the periodic table; anything beyond period 2 is unlikely to be stable with fewer than eight electrons in their outer shell.
How do expanded octets form?
The ability of a central atom to achieve stability with an expanded octet can be linked to its position on the periodic table; it is only possible for an element in period 3 or below.
These atoms have empty 3d-orbitals which, in terms of energy, are very close to the 3p-orbitals. This makes it possible to promote electrons from the 3p- to the 3d-orbital if needed, which allows additional electron pairs to form.
Steps to draw a Lewis formula for molecules and ions that have central atoms with an expanded octet
Calculate the total number of valence electrons in the molecules. Adjust for the charge if required.
Identify the central atom. Draw the basic structure of the molecule by linking the peripheral atoms to the central atom with single bonds.
Add remaining electrons (following the octet rule).
If there are not enough electrons to complete the octets, form double and triple bonds if necessary.
Check that the total number of electrons in the finished structure is equal to your calculation in the first step.
If all peripheral atoms have fulfilled the octet rule and there are still electrons remaining, add them as pairs to the central atom, provided the central atom is from period 3 or below.
What is formal charge?
The theoretical charge of an entire molecule based on the electron distribution of the atoms within the molecule. (which one is more likely)
How can we determine he stability based on the formal charges
The most likely structure to exist is mostly the one that is always the one with the forml charge closest to 0.
This is because a low formal charge means that less charge transfer has taken place in forming the structure, indicating that the number of valence electrons before and after bonding is similar. This results in a more stable structure overall.
How to calculate the formal charge?
Formal charge= Number of valance electrons - ( Number of electrons assigned to atom in Lewis structure so bonding and lone airs of electrons in a Lewis structure)
FV= V - (B+L)
B= bonding pairs
L= lone ELECTRONS
How is formal charge affected by ions?
. In an ion, the sum of the formal charges must equal the charge of the ion, reflecting the number of electrons that have been gained or lost.
not all atoms in the molecule or ion will have a formal charge of zero: the goal is to have as many as possible with a formal charge of zero.
Limitations for formal charge
It does not consider electronegativity (because its not based on their properties)
What is a sigma bond?
Sigma bonds involve a head-on overlap of atomic orbitals (s and s, s and p, p and p).
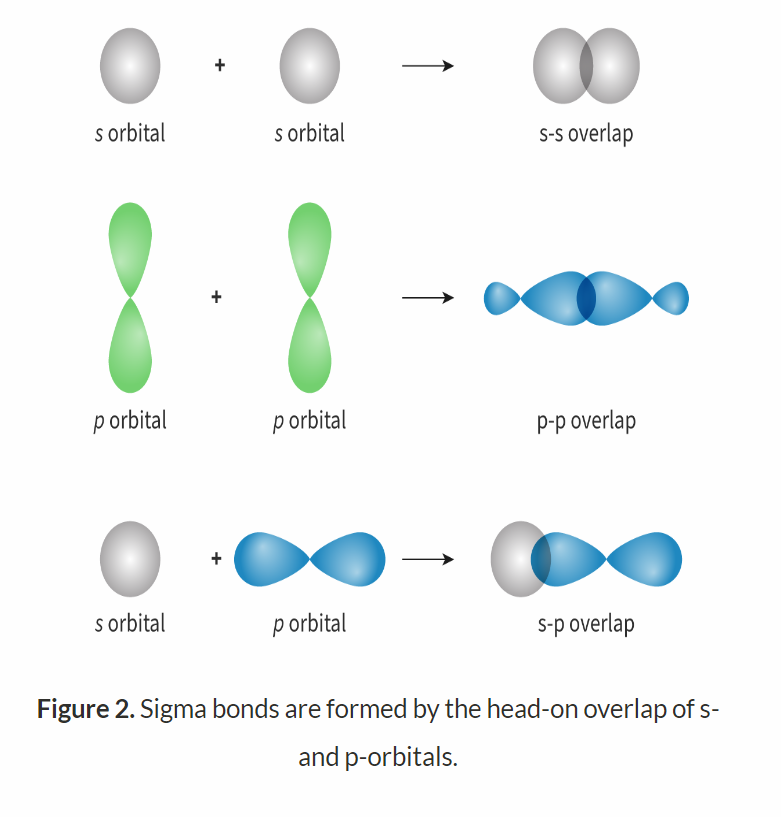
What is a pi bond?
Pi bonds result from the sideways overlap of p orbitals.
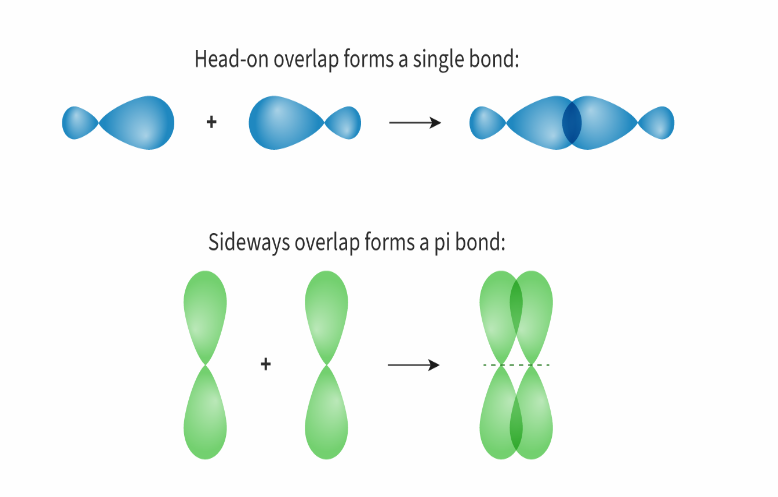
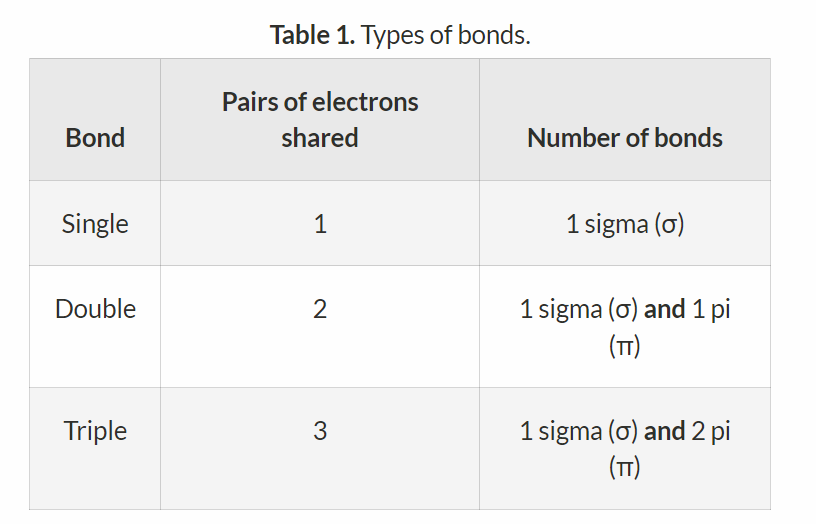
In what type of bonds how many sigma and pi bonds exist?
Compare pi bonds and sigma bonds in terms o bond angles
Bond rotation is possible in a sigma bond as there is only one region of electron density, located along the inter-nuclear axis. As a pi bond has two regions of electron density, located above and below the plane of the inter-nuclear axis, it is not possible for a pi bond to rotate.
Compare pi bonds and sigma bonds in terms of strength
Sigma bonds are stronger than pi bonds.
A sigma bond has a stronger attraction between the electron pair and positive nuclei as there is only one electron-dense region on the inter-nuclear axis, located close to the nuclei
In a pi bond the electron density is spread across two regions, above and below the inter-nuclear axis, and is found further from the positive charge of the nucleus.
Relationship between resonance and pi bonds
the electron which dissociates to maintain the resonance is from the pi bond.
bonding and structure in benzoic acid, C6H5COOH.
Number of electron domains per carbon: 3
Sigma (σ) bonds: 15
Pi (π) electrons: 8
Explanation
Each carbon atom is bonded to a hydrogen atom with a sigma bond.
Each carbon atom is also bonded to two other carbon atoms (one on each side) with a sigma bond, and there is a pi bond spread across two bonding positions.
Single bonds are all sigma bonds.
Double bonds all contain one sigma and one pi bond.
What is hybridisation ?
the mixing of orbitals to produce hybrid orbitals used for bonding
the total energy of the orbitals does not change, it redistributes equally among four new hybrid orbitals that are now at intermediate energy level. The hybridisation makes the molecule more stable.
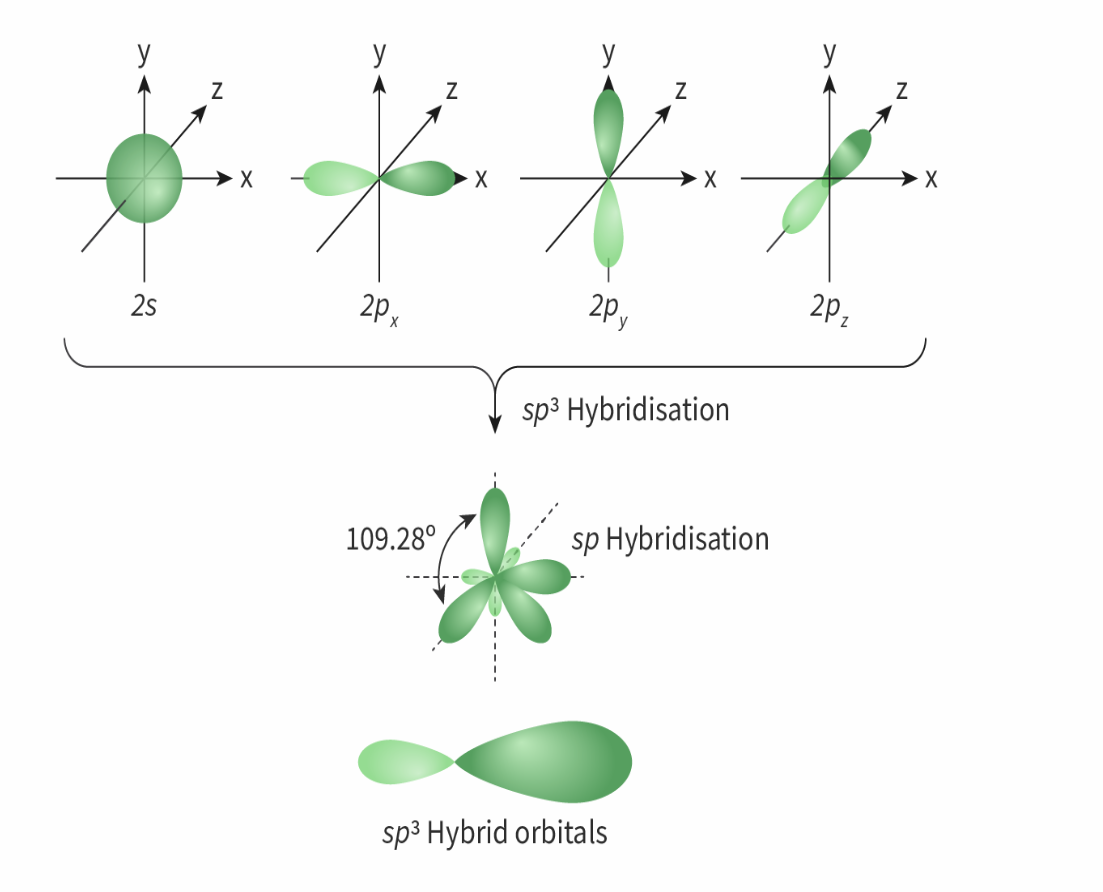
sp³ Hybridisation molecular geometry
Number of electron domains: 4
Electron geometry: tetrahedral
Molecular geometry: Tetrahedral
Bond angle :< 109.5
e.g: CH4, H2O, NH3
always forms four sigma bonds
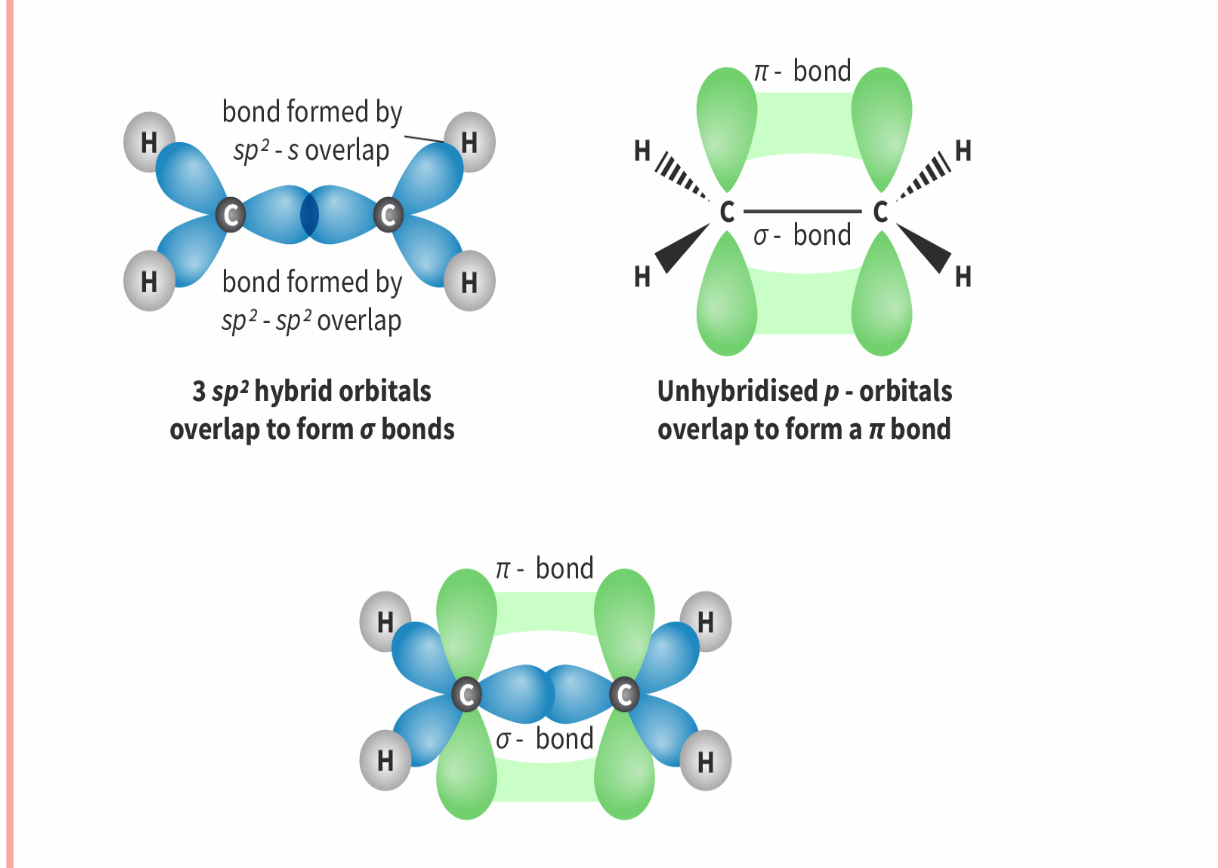
sp² Hybridisation geometry
Number of electron domains: 3
Electron geometry: trigonal planar
Molecular geometry: Trigonal planar
Bond angle :< 120
e.g: C2
one s-orbital and two p-orbitals would become hybridised to produce three equal orbitals. One of the 2p-orbitals is left unhybridized which forms the pi bond (as part of the double bond) .
The unhybridised p-orbitals that overlap sideways in the pi bond keep the same dumb-bell shape.
three sigma bonds and one pi bond
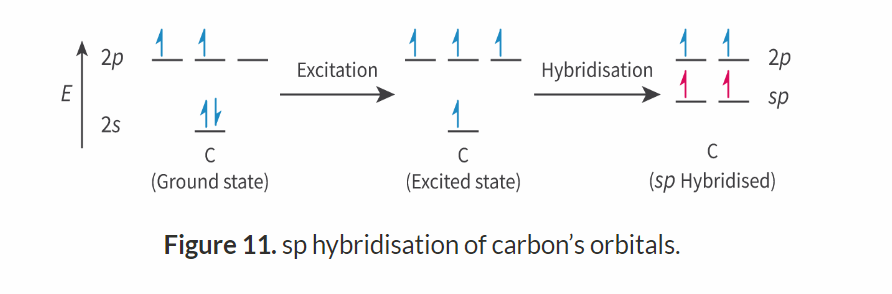
sp Hybridisation
Number of electron domains: 2
Electron geometry: linear
Molecular geometry: linear
Bond angle :180
e.g: C2H2
It will only hybridise two of its orbitals, one of the s-orbitals and one of the p-orbitals. produces equal orbitals. The other two p-orbitals are left unhybridized
What is the hybridisation of benzene
As both single, double and triple bonds are all counted as one electron domain, the presence of delocalised electrons does not change how many electron domains surround each carbon, each carbon still has three electron domains. Therefore, the hybridisation experienced by carbon in benzene is sp2