Unit 1: Atomic structure, Electronegativity, Bonding, and Formal charges
0.0(0)
Card Sorting
1/3
Earn XP
Last updated 3:11 AM on 1/15/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
1
New cards
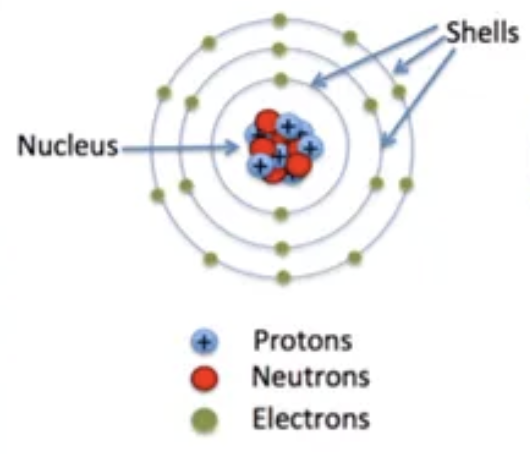
Atoms are made of what?
Atoms are made of protons, electrons, and neutrons.
2
New cards
Electrons surrounds?
Electron surrounds the nucleus (containing protons and neutrons) in “shells”
3
New cards
Shells are ___?
Shells are quantized, which means that each shell has its own specific value of energy.
4
New cards
What are subshells called and some examples?
Subshells are called orbitals, and examples include s, p, d, and f orbitals. Subshells are within other shells