1.6 - Graphenes, Fullerenes and Nanotubes
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
What type of structures are graphenes, fullerenes and nanotubes?
giant covalent structures
What is graphene?
single sheet of hexagonal rings of carbon atoms
How thick is grphene?
only one atom thick
Wht is graphene a single layer of?
graphite
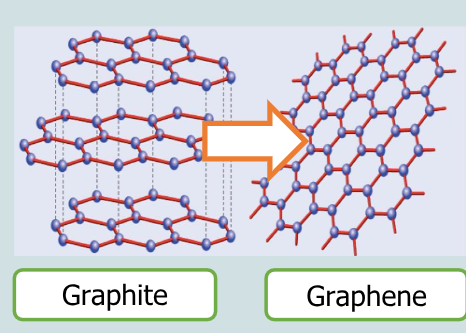
What dimenson is graphene?
the worlds first 2d material
What are the properties of graphene?
• excellent thermal and electrical conductor
• very low density (lightweight)
• flexible
• transparent
• most reactive form of carbon
• very strong for its mass (over 200x stronger than steel)
What are the potential uses of graphene
Quicker and more powerful computer chips, electric circuits and solar cells
Flexible and durable electronic display screens
What is an allotrope?
a new form of an element
When was Buckminister Fullerene discovered?
1985
Have other fullerenes been discovered since?
Yes
What are the names of other fullerenes that have been made?
C70, C76 and C84
What are fullerenes?
molecules of carbon atoms joined together to make hollow cages of different shapes, usually hexagonal rings of carbons atoms but can be pentagonal or heptgonal
What are Buckminister Fullerenes made of?
molecules of C60
What bonds are between each carbon atom in a Buckminister Fullerene?
strong covalent bonds
How is each fullerene molecule attracter to each other?
weak intermolecular forces
How big is the fullerene molecule?
1nm in diameter
What are the uses of fullerene?
Used as cages to trap smaller molecules inside them.
Used to carry drug molecules around the body
Used as lubricants - Fullerenes are spherical with weak intermolecular forces so can roll over each other
What are nanotubes?
hollow, cylindrical tubws of carbon atoms
What is the structure of nanotubes?
giant covalent strucutre where each carbon atom forms 3 bonds to other carbon atoms
What are the properties of nanotubes?
very strong/high tensile strength
very good electrical and thermal conductors
Why are nanotubes good conductors?
Each C atom has one electron not involved in bonding / that becomes delocalised which can move freely through the structure, carrying charge
What are the uses of nanotubes?
can be used to reinforce materials like tennis rackents due to their strength
can be used as catalysts because they have a large surface area to volume ratio