Chapter 6 - Shapes of Molecules + Intermolecular Forces
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
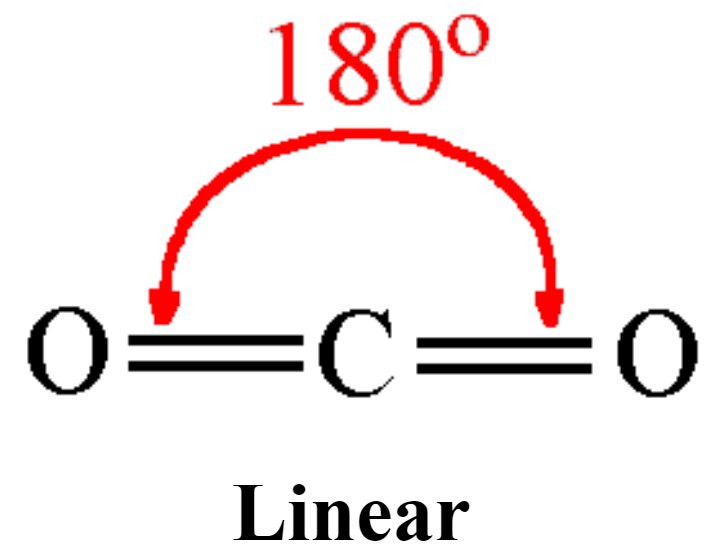
LINEAR shape
bond angle = 180, has 2 bonding pairs
example = carbon dioxide
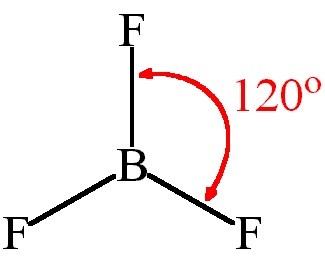
TRIGONAL PLANAR shape
bond angle = 120, has 3 bonding pairs
example = boron trifluoride
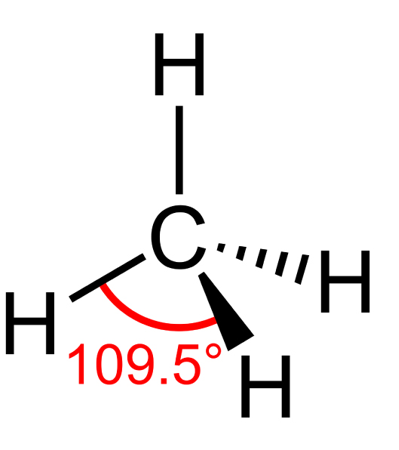
TETRAHEDRAL shape
bond angle = 109.5, has 4 bonding pairs
example = methane
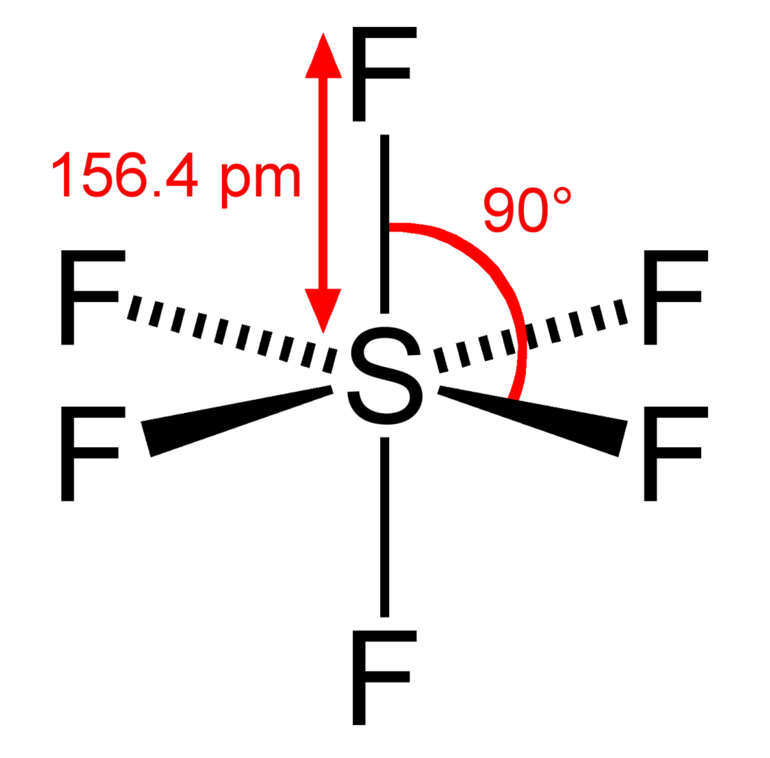
OCTAHEDRAL shape
bond angle = 90, has 6 bonding pairs
example = sulfur hexafluoride
how many degrees do lone pairs decrease the bond angle?
2.5
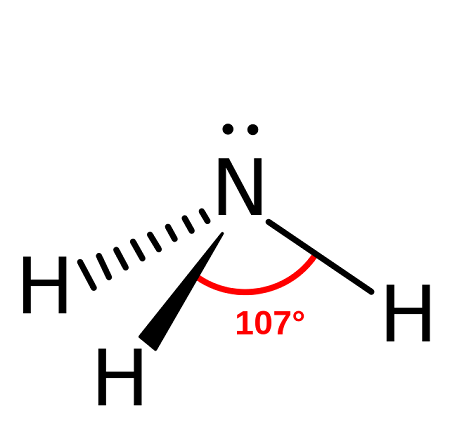
PYRAMIDAL shape
bond angle = 107, has 3 bonding pairs, has 1 lone pair
example = ammonia
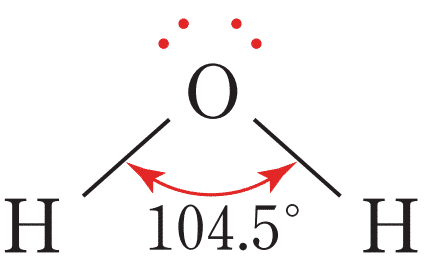
NON-LINEAR shape
bond angle = 104.5, has 2 bonding pairs, has 2 lone pairs
example = water
electronegativity
the ability of an atom to attract bonding electrons in a covalent bond
TRENDS in electronegativity -period
electronegativity increases
-nuclear charge increases
-atomic radius decreases
-electron shielding remains the same
-nuclear attraction experienced by bonding electrons increases
TRENDS in electronegativity -group
electronegativity decreases
-atomic radius increases
-increase in nuclear charge is outweighed by increase in electron shielding
-nuclear attraction decreases
permanent dipole
there is a small charge difference across a bond resulting in a large difference in electronegativity (>1.4) of the bonded atoms
polar covalent bond
-when a covalent bond contains a permanent dipole
-the bonded electron pair is shared unequally as the bonded atoms are different and have different electronegativity values so one element is more electronegative creating the dipoles
covalent bond with no permanent dipole
-the bonded electron pair is shared equally as the bonded atoms are the same and have similar/the same electronegativity values
polar molecule
-has an overall dipole
-the permanent dipoles do not cancel each other out because the bonds are arranged asymmetrically as they act in different directions
non-polar molecule
-no overall dipole
-the bonds are arranged symmetrically so the dipoles act in equal and opposite directions so cancel each other out as they oppose each other
TYPES of intermolecular forces
1) London forces (induced dipole-dipole)
2) permanent dipole-dipole interactions
3) hydrogen bonding
-also in increasing strength order
London forces
-weakest type of intermolecular force
-exists between all molecules (polar or non-polar)
STRENGTH of London forces
the greater the number of electrons in the molecule, the larger the induced dipoles so the stronger the attractive forces between the molecules so the melting and boiling point increase as more energy is needed to overcome
permanent dipole-dipole interactions
-exist in polar molecules that contain permanent dipoles
-are stronger and never change unlike London forces but not as common
-S+ region of one molecule is attracted to S- region of another molecule
hydrogen bonding
-need very large difference in electronegativity to exist → fluorine, nitrogen and oxygen are the most electronegative
-strong intermolecular forces formed between molecules containing N-H, F-H AND O-H
effects of HYDROGEN BONDING on WATER 1)
ice is less dense than liquid water
-in solid ice, the water molecules are held apart by hydrogen bonds so causes it to have an open lattice structure
-when heated, the hydrogen bonds break and the water molecules can move closer together so liquid water is denser
effects of HYDROGEN BONDING on WATER 2)
water has a relatively high melting/boiling point
-has hydrogen bonding which is stronger than other intermolecular forces so more heat energy required to overcome