cell signalling
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
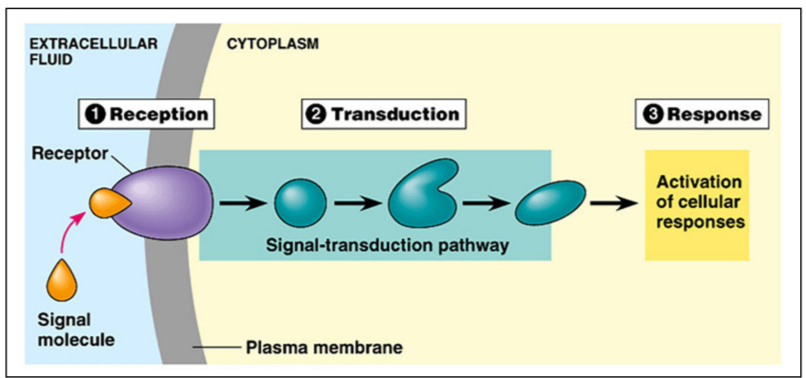
Outline the main stages of cell signaling
Ligand-receptor interaction
The ligand is complementary in shape to a specific binding site on the receptor and attaches itself there.
The binding of the ligand to the receptor induces a conformational change in membrane-bound receptor. This change in shape activates the receptor, triggering downstream signalling pathway.
The activated receptor then interacts with another molecule or dimerize with another receptor molecule.
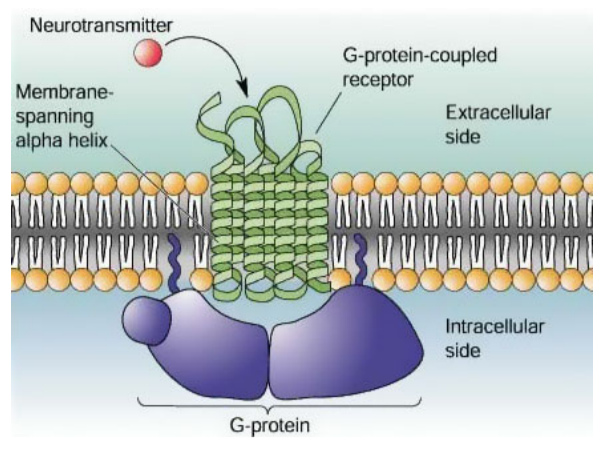
Structure of GPLR (G protein-linked receptor)
A protein that binds to GTP or GDP.
Extracellular region: Ligand binding site
Intracellular region: G-protein binding site
Transmembrane region: 7 hydrophobic transmembrane a-helices
Structure of RTK (Receptor Tyrosine Kinase)
Extracellular region: Ligand binding site
Intracellular region: Tail that functions as tyrosine kinase and also contains a number of tyrosine a.a residues
Transmembrane region: A single polypeptide chain with a single transmembrane a-helix
Signal transduction
Phosphorylation cascade
Activated receptor activates a relay molecule, which activates another relay molecule, and so on, until the molecule that produces the final cellular response is activated
Protein kinases are enzymes that transfer phosphate groups from ATP to protein, ie phosphorylation
Protein phosphatases are enzymes that remove phosphate groups from proteins, ie dephosphorylation
Each activated protein kinase will initiate a sequential phosphorylation and activation of other kinases, resulting in a phosphorylation cascade
Relay molecules are usually activated when they are phosphorylated and deactivated when they are dephosphorylated.
Signal amplification
This relaying of signals allows for greater fine-tuning of cellular responses and for amplification of the signal
Each catalytic step in a cascade produces a larger number of activated products than in the preceding step.
Thus, a very small amount of signal will give a large response as each activated enzyme molecule can convert many substrate molecules into products per unit time before being inactivated.
Cellular response (hpnestly very dependent on the context of the question)
eg regulation of activity of protein (opening / closing of an ion channelin the CSM to change membrane permeability)
eg regulation of ysnthesis of protein by turning a specific gene expression on or off in the nucleus
eg apoptosis
eg enzyme regulation etcetc
Explain the roles and nature of second messengers
Nature
Small
Non-protein
Water-soluble
Role
Relay signals received at the receptors on the cell surface to target molecues in the cytosol and / or nucleus + amplify the strength of signals
Cyclic adenosine monophosphate (cAMP)
Adenylyl cyclase, an enzyme embedded in the CSM, when activated by the G protein, can convert many ATP to cAMP molecules.
The cytosolic concentration of cAMP is elevated twenty-fold in a matter of seconds, amplifying the signal in the cytoplasm.
It does not persist for long in the absence of the hormone, because another enzyme, phosphodiesterase, converts cAMP to AMP, resulting in signal termination.
Explain the role of kinases and phosphatases in signal amplification.
Kinase are enzymes that add phosphate groups to proteins using phosphates from ATP, which in turn activates these proteins.
Since kinase are enzymes, they can phosphorylate many kinases resulting in signal amplification.
If a kinase enzymes activate another kinase enzyme, it results in a phosphorylation cascade
Phosphatases remove phophate group from proteins to inactivate them
Outline how glucagon regulates concentration of blood glucose through GPLR.
Signal: Glucagon
Purpose: When blood glucose level falls below the set point (e.g during fasting), glucagon is released to activate different transduction pathways to brung about the same set of cellular responses that release glucose back to the blood to restore set point
Receptor: GPLR
Target cells: Liver cells
Ligand-receptor interaction
Glucagon binds to extracellular site of GPLR → activates the receptor → change in conformation
Cytoplasmic side of GPLR binds an inactive G protein → G protein exchanges bound GDP for GTP
G protein is activated & dissociates form the receptor.
Activated G protein binds and activates adenylyl cyclase → catalysing the converstion of large number of ATP to cAMP.
Transduction
cAMP (second messenger), binds and activates a large number of protein kinase A (PKA)
Each activated PKA will initate a sequential phosphorylation & activation of other kinases → phosphorylation cascade
At each phosphorylation step, each activated kinase is able to activate a large number of the next kinase
At each catalytic step in the cascade, the number of activated product is always greater than those in the preceding step → signal amp
Final protein to be activated is glycogen phosphorylase
Reponses
A large number of glycogen phosphorylase is activated → will catalyse the breakdown of glycogen into glucose via glycogenolysis
Increased synthesis or activity of enzymes involed in glucose synthesis via gluconeogensis.
INCREASED gluconeogenesis and glycogenolysis → DECREASED glycolysis and glycogenesis (sts of glycogen from glucose for storage)
Blood glucose concentration back to set-point.
Outline how insulin regulates concentration of blood glucose through RTKs
Signal: Insulin
Purpose: When blood glucose level rises above a set point, insulin released by the B cells of Islets of Langerhans of pancrease lowers blood glucose level back to the set point.
Receptor: RTK
Target cells: Liver cells, muscle cells, other respiring cells
Ligand-receptor interaction
Binding of insulin to extracellular binding sites of RTK → 2 RTK protein to form a dimer
Dimerisation actives tyrosin kinase function found in intracellular tails of RTK
Tyrosine kinase adds a phosphate group from ATP molecule to tyrosin residues on the tail of other RTK protein by cross-phosphorylation
Transduction
Activated RTK trigger assembly or relay proteins on receptor tails → activating them
Activated relay proteins will further recruit and activate other downstream relay molecules and protein kinases
Each activated protein kinase will initiate a sequential phosphorylation and activation of other kinases, resulting in phosphorylation cascade.
At each phosphorylation step, each activated kinase is able to activate a large number of the next kinase.
At each catalytic step in the cascade,the number of activated product is greater than those in the preceding step → signal amplification
Response
INCREASE in rate of processes that remove glucose from blood
Eg; ↑ transport of glucose transporters to cell surface membrane to increase glucose uptake into the cell
↑ glycogenesis (synthesis of glycogen from glucose for storage)
↑ glycolysis (oxidation of glucose), hence increased ATP synthesis
↑ fatty acid synthesis
DECREASE in rate of processes tha return glucose to the blood
Eg; ↓ glycogenolysis (hydrolysis of glycogen to glucose)
↓ gluconeogenesis (conversion of non-carbohydrate sources to glucose)
Blood glucose concentration is DECREASED to set point