energy changes topic 5
1/8
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
9 Terms
how much energy can chemical store take in, if products of reaction store more energy then, if they store less, why doesnt the overall amount of energy change
chemicals store certain amount energy & diff chemicals store diff amounts
if products of reaction store more energy than og reactants, then must have taken in diff in energy between products & reactants from surroundings during reaction
if store less, then excess energy was transferred to surroundings during reaction
overall amount energy doesn’t change because energy is conserved in reactions → cant be created, destroyed only moved around = energy in universe stays same
whats exothermic reactions & hows it shown, 2 examples, how is oxidation reactions sometimes exothermic, 2 everyday uses of it
exothermic reaction → transfers energy to surroundings usually by heating, shown by rise in temp
example → burning fuels or sometimes called combustion = gives out loads energy, very exothermic
neutralisation reactions (acid + alkali) r also exothermic
many oxidation reactions r exothermic → adding sodium to water releases energy = exothermic. The reaction releases energy & sodium moves about on surface of water as its oxidised
exothermic reactions have lots everyday uses
some hand warmers use exothermic oxidation of iron in air (w salt solution catalyst) to release energy
self heating cans of hot chocolate & coffee also rely on exothermic reactions between chemicals in their bases
whats endothermic reaction & hows it shown, they r…, 2 examples, everyday use
endothermic reaction → takes in energy from surroundings, shown by fall in temp
much less common than exothermic reactions but they include:
reaction between citric acid & sodium hydrogencarbonate
thermal decomposition - heating calcium carbonate causes it to decompose into calcium oxide (quicklime) & carbon dioxide
CaCo_3 (+heat) → CO_2 + CaO
endothermic reaction everyday uses:
used in some sports injury packets - chemical reactions allows pack to become instantly cooler without having to put it into freezer
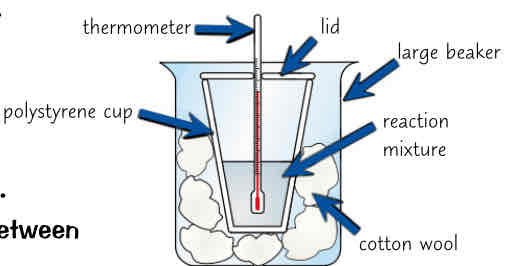
how can u measure amount of energy released by chemical reactions, biggest prob w energy measurements…, how to reduce this, what else can we measure w this method
can measure amount of energy released by chemical reaction (in solution) by taking temp of reagents, mixing them in polystyrene cup & measuring temp of solution at end of reaction
biggest problem w energy measurements is amount energy lost to surroundings
can reduce it a bit by putting polystyrene cup into beaker of cotton wool to give more insulation & putting lid on cup to reduce energy lost by evaporation
can also use method to investigate what effect diff variables have on amount of energy transferred (mass/ concentration of reactants used)
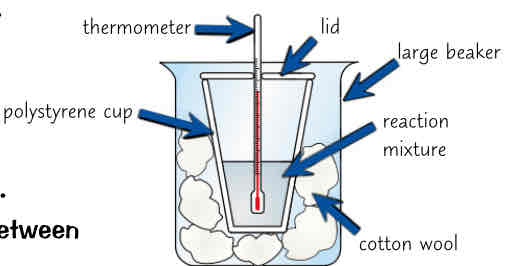
how could u test effect of acid concentration on energy released in neutralisation reaction between hydrochloric acid (HCl) & sodium hydroxide (NaOH) (5 steps)
put 25cm³ of 0.25mol/dm³ of hydrochloric acid & sodium hydroxide in separate beakers
place beakers in water bath set to 25°C until both r at same temp
add HCl followed by NaOH to polystyrene cup w lid
take temp of mixture every 30 seconds & record highest temp
repeat steps 1-4 using 0.5mol/dm³ & then 1mol/dm³ of hydrochloric acid
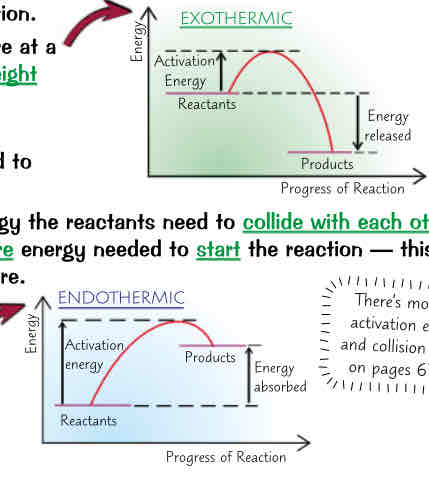
whats reaction profiles, exo: y is it an exothermic reaction profile, diff in height, intial rise in energy reps, whats activation energy, greater activation energy…, endo: whys it endothermic reaction profile, diff in height reps
reaction profiles → diagrams that show relative energies of reactants & products in reaction & how energy changes over course reaction
exothermic reactions:
products at lower energy than reactants
diff in height reps overall energy change in reaction (given out) per mole
initial rise in energy reps energy needed to start reaction → activation energy (E_a)
activation energy is minimum amount of energy reactants need to collide w each other & react
greater activation energy = more energy needed to start reaction (has to be supplied by heating reaction mixture)
endothermic reactions:
products at higher energy then reactants
diff in height reps overall energy change during reaction (energy taken in) per mole
what happens in some cells, what happens over time to reacting particles, why is no electricity produced, what r non-rechargeable batteries (definition), what can happen in rechargeable cell
in some cells chemical reactions happen at electrodes r irreversible
over time reacting particles - ions in electrolyte & metal ions on electrode - get used up & turned into products of reaction
once any one of reactions is used up the reaction cant happen & so no electricity is produced
non-rechargeable batteries → alkaline batteries, contain cells which use irreversible reactions, once one of reactants is used up they dont produce any more charge & have to replace them
in rechargeable cell, reaction can be reversed by connecting it to an external electric current
whats fuel cell, what happens when fuel enters cell, what can diff fuel cells use, important example & what does it produce
fuel cell → electrical cell thats supplied w fuel & oxygen (/ air) & uses energy from reaction between them to produce electrical energy efficiently
when fuel enters cell it becomes oxidised & sets up pd within cell
few diff types of fuel cells, using diff fuels & diff electrolytes,
important example → hydrogen-oxygen fuel cell, combined hydrogen & oxygen to produce nice clean water & release energy
3 cons of using hydrogen-oxygen fuel cells in vehicles
hydrogen is gas so it takes up loads more space to store than rechargeable battery
hydrogen is explosive when mixed w air so its hard to store safely
hydrogen fuel is often made either from hydrocarbons (from fossil fuels) or by electrolysis of water which uses electricity (& electricity has got to be generated somehow - usually fossil fuels)