BIOL 1020: Lectures 24,25,26 - Catabolic Pathways
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
Compare catabolism and anabolism
Catabolic pathways
release energy by breaking down complex molecules into simpler compounds
depend on the transferring of electrons during chemical reactions
Aerobic respiration
oxygen is consumed as a reactant along with organic fuel
Anaerobic respiration
organic fuel is broken down wihtout oxygen
Fermentation
partial breakdown of organic fuel without oxygen
Explain redox reactions
Reduction-Oxidation
the transfer of electrons from one molecule to another
LEO GER
Reducing agent
molecule that gives up electrons and becomes oxidized
oxidized: electrons are further
Oxidizing agent
molecule that receives electrons and becomes reduced
reduced: electrons are closer
Nicotinamide adenine dinucleotide (NAD+)
an electron transporter
facilitates the electron transfer over multiple steps in the breakdown of glucosse
a coenzyme and oxidizing agent
it can cycle between an oxidized (NAD+) and reduced (NADH) form
NADH just carries e- without releasing as much energy
What would happen if the transfer of electrons was uncontrolled?
one big release of energy with lots of heat loss
What would happen if the transfer of electrons was controlled?
small releases of energy at each step, which can be used to make more ATP
electrons get removed from glucose and are transfered to the ETC via NADH
the bondss that hold the e- in the ETC are increasingly unequal
Substrate level phosphorylation
an enzyme catalyzes the transfer of a phosphate group from a substrate to ADP, forming ATP
the substrate is generated as an intermediate in the breakdown of glucose
direct transfer of energy to ATP
accounts for about 10% of ATP generation during cellular respiration
Occurs in cytosol and mitochondriia
Oxidative phophorylation
energy dissipated from electrons in the ETC makes an H+ gradientt
this gradient is used to drive a protein complex called ATP synthase
indirect transfer of energy to ATP
Makes aprox. 90% of ATP generated in cellular respiration
Occurs only in the mitochondria
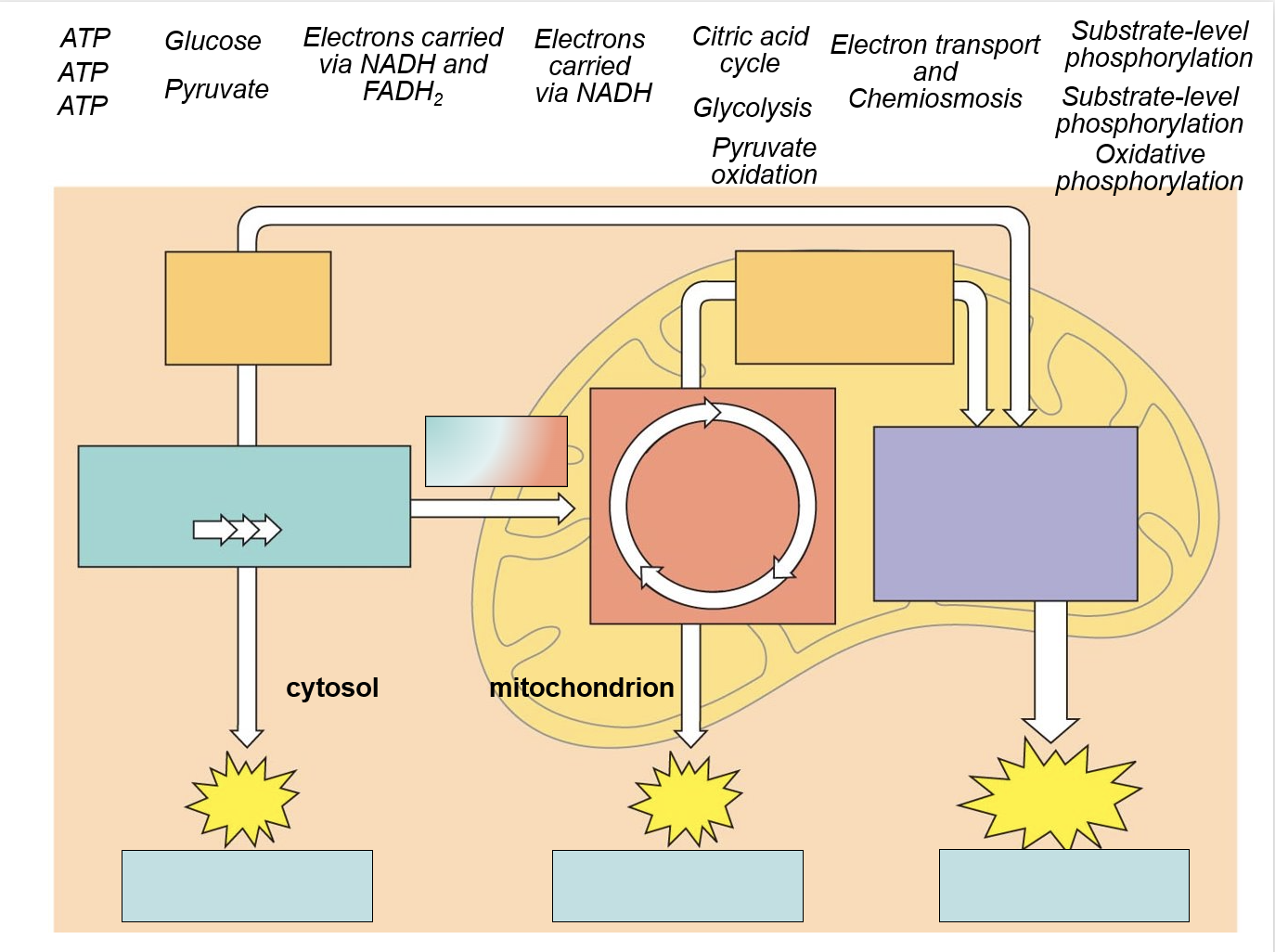
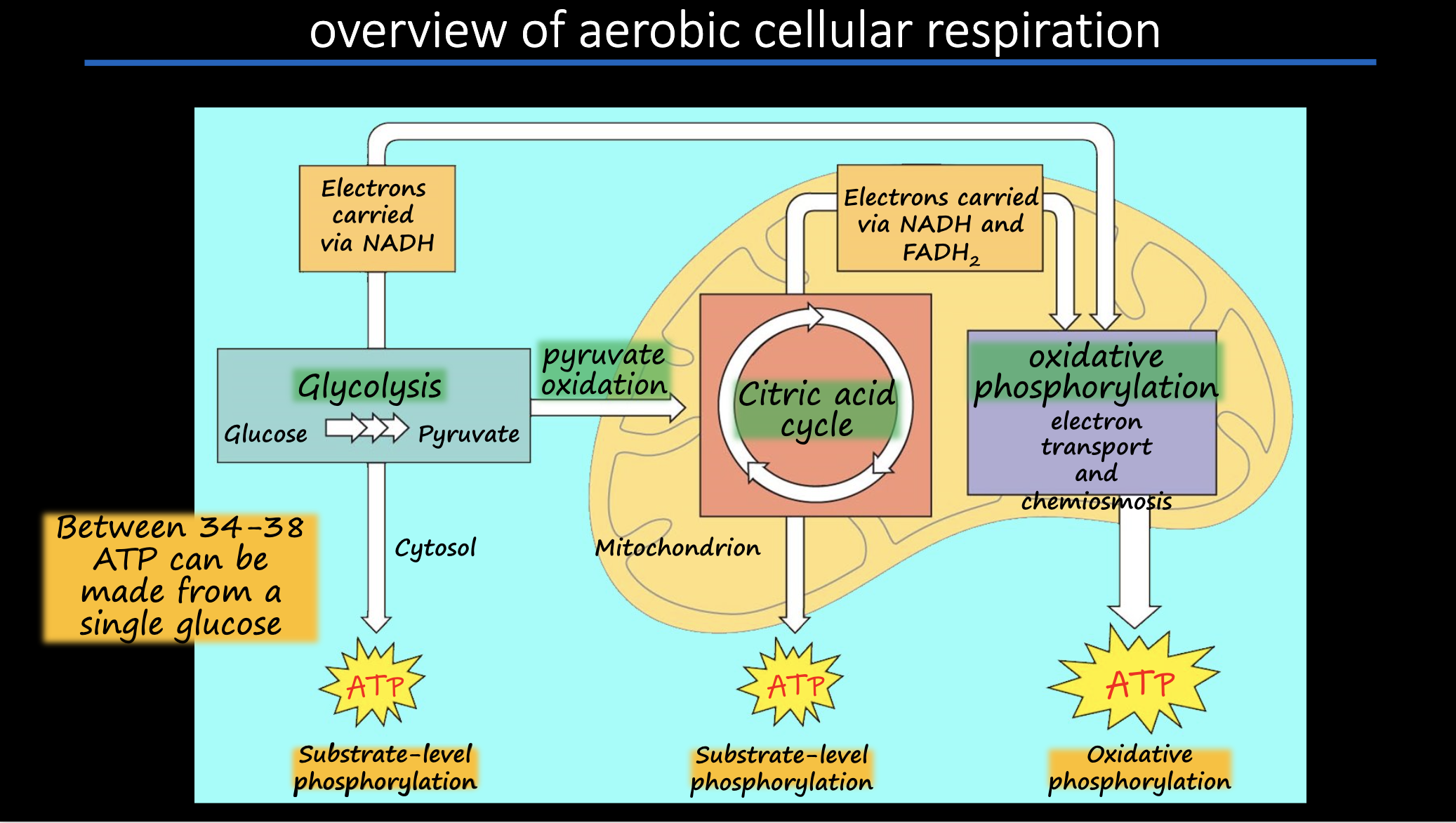
Glycolysis
sugar spltting: glucose (6C sugar) is split into two 3C sugars
the 3C sugars are then oxidized and rearranged to form 2 pyruvates used in the next steps of cellular respiration
Note:
Carbon is not loss
Glycolysis occurs with or without oxygen; if oxygen is present, it will proceed into the rest of aerobic respiration
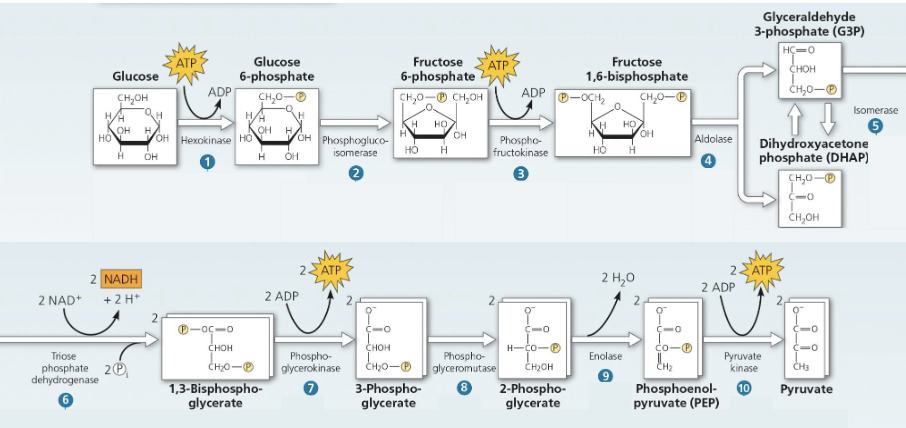
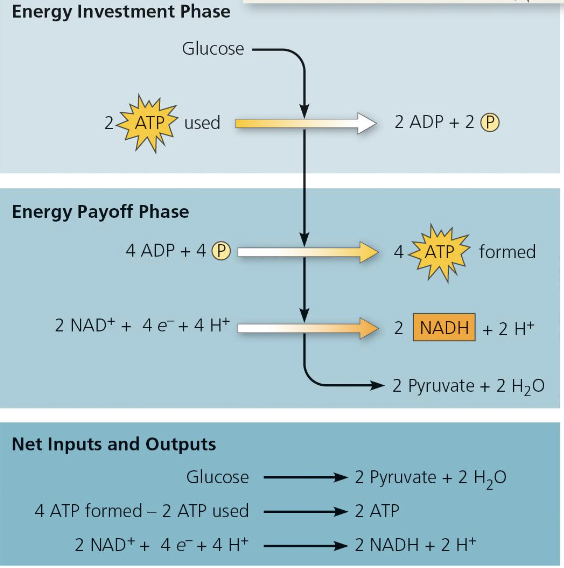
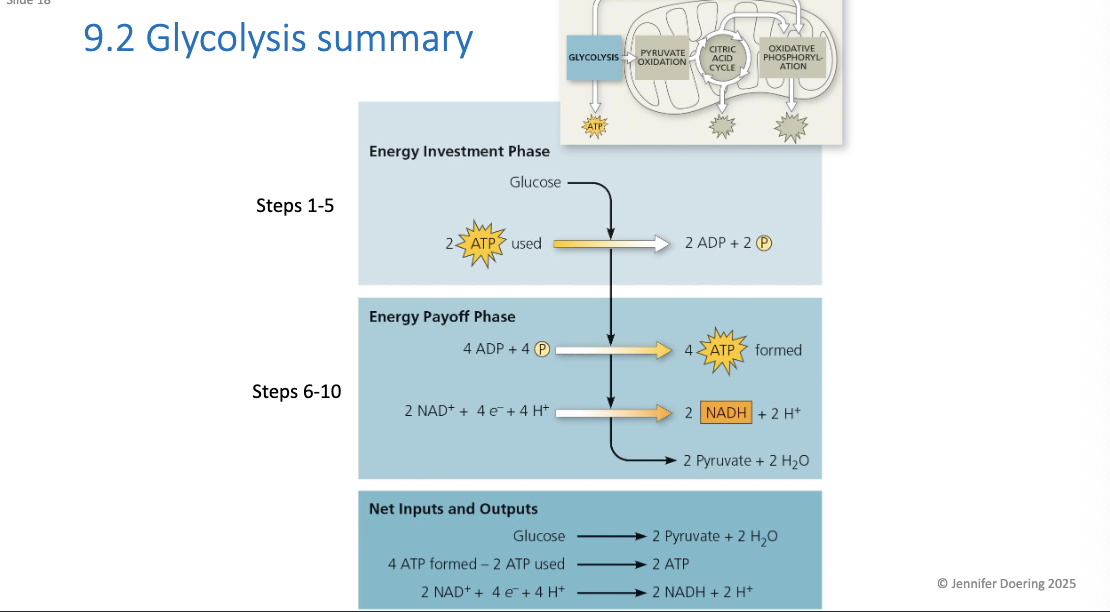
Energy investment and Payoff
Investment: the cell spends 2 ATP
Payoff: 4 ATP is produced via substrate level phosphorylation
Overall: Net gain of 2ATP + 2NADH
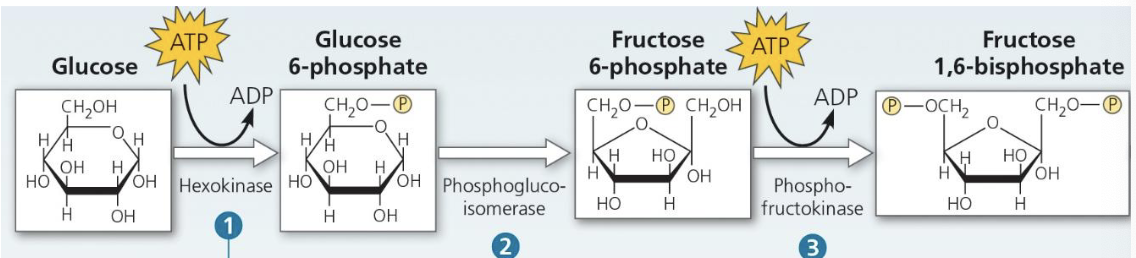
Explain what’s happening here
Glucose is phosphorylated by ATP by hexokinase — makes it more chemically reactive
Glucose 6-phosphate is converted into fructose-6-phossphate
Phosphofructokinase transfers another phosphate to other end of fructose 6-phosphate,making fructose 1,6-biphosphate (2nd ATP investment)
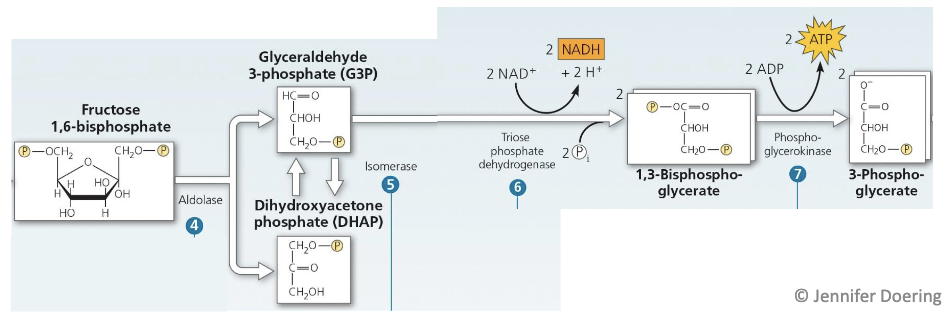
Explain what’s happening here
Aldolase cleaves fructose 1,6-biphosphate into two different 3C sugars
Dihydroxyacetone phosphate (DHAP) → used in adipose cells to generate glycerol backbone
Glyceraldehyde 3-phosphate (G3P) → moves into citric acid cycle
G3P and DHAP are converted back and forth as they are produced, but the reaction never reaches equilibrium as G3P is used up almost as fast as it is produced
G3P is oxidized by transferring electrons to NAD+, forminig NADH, and the energy from this exergonic reaction is used to phosphorylate the oxidized substrate
The phosphate group is transfered to ADP in an exergonic reaction (Substrate level phosphorylation), leaving the carbonyl group of G3P oxidized to a carboxyl group of 3-phosphoglycerate
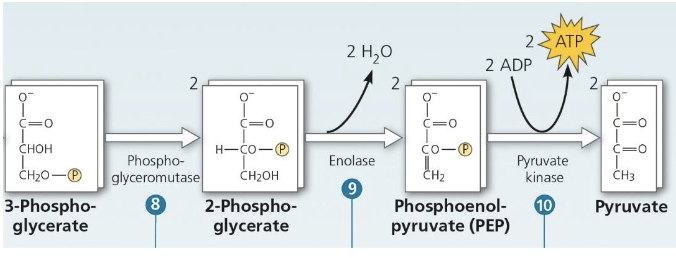
Explain what’s happening here
Phosphoglyceromutase relocates the remaining phosphate group
Enolase causes a double bond to form in the substrate by extracting a water molecule, which makess phossphoenolpyruvate (PEP) → high energy potential
The phosphate group is transferred from PEP to ADP (substrate level phosphorylation), forming pyruvate
Pyruvate is transferred to the citric acid cycle
What happens after glycolysis
pyruvate is actively transported into the mitochondrion
pyruvate is then converted into acetyl CoA via three steps by a pyruvate dehydrogenase enzyme complex
The oxidizing carboxyl group on pyruvate iis removed
releases CO2; is now a 2C molecule
the 2C molecule is oxidized, formiing acetate (CH3COO-)
extracted e- are transferred to NAD+, forming NADH (stores NRG)
Coenzyme A (S-CoA) attaches by its sulfur group
The citric acid cycle
generates 1 ATP molecule per cycle via SLP
Most energy is transferred to NAD+ and FAD
FAD: flavin adenine dinucleotide; from riboflavin
FADH2 = reduced form
FAD = oxidized form
These shuttle electrons to the ETC where most of the energy will be produced
8 main steps once acetyl CoA enters the cycle
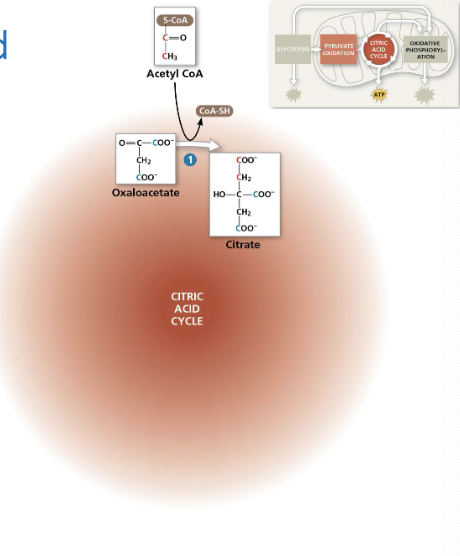
Acetyl CoA adds its 2 carbon acettyl group to 4C oxaloacetate, producing 6C citrate
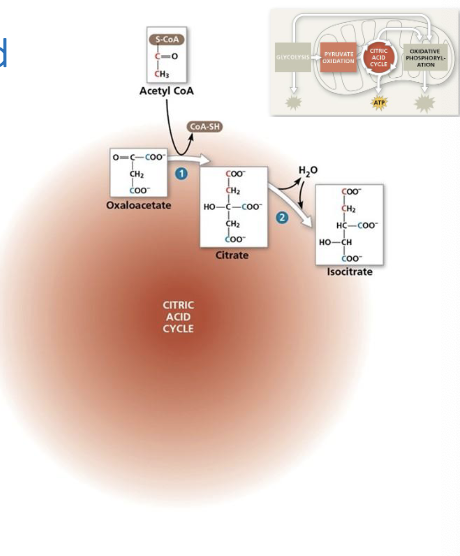
Citrate is converted to its isomer, isocitrate (6C), by dehydration (removal of water) but also the addition of water
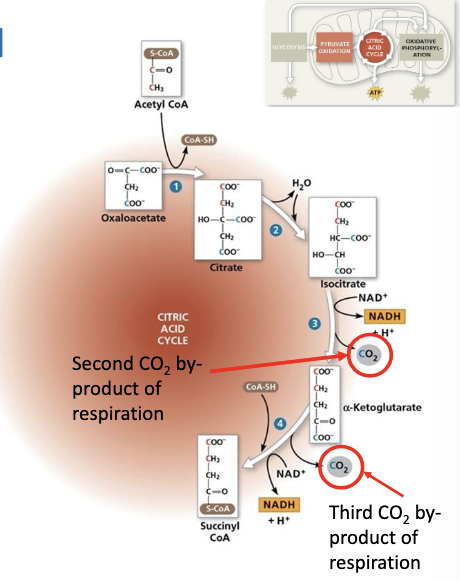
Isocitrate is oxidized, reducing NAD+ to NADH. A CO2 molecule is lost, results in alpha-ketoglutarate (5C)
Another CO2 is losst, and the resulting compound is oxidized, transferring electrons to NAD+ form NADH
the remaining molecule then bondss with coenzyme A, resulting in the formation of Succinyl CoA (4C) which has very unstable bonds
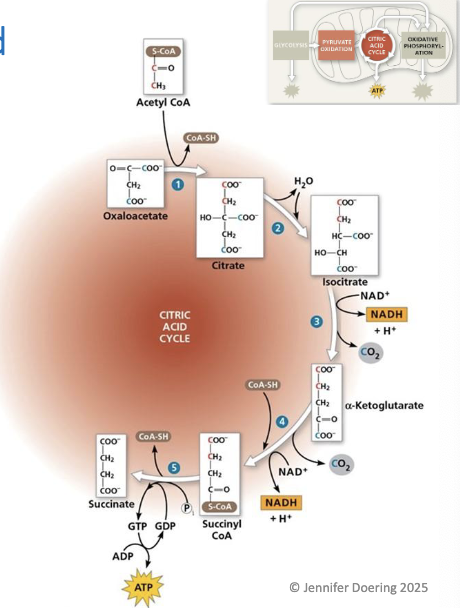
CoA group is displaced by a phosphate group, which is transferred to GDP, forming GTP
GTP
can be used to indirectly produce ATP
results in the production of succinate (4C)
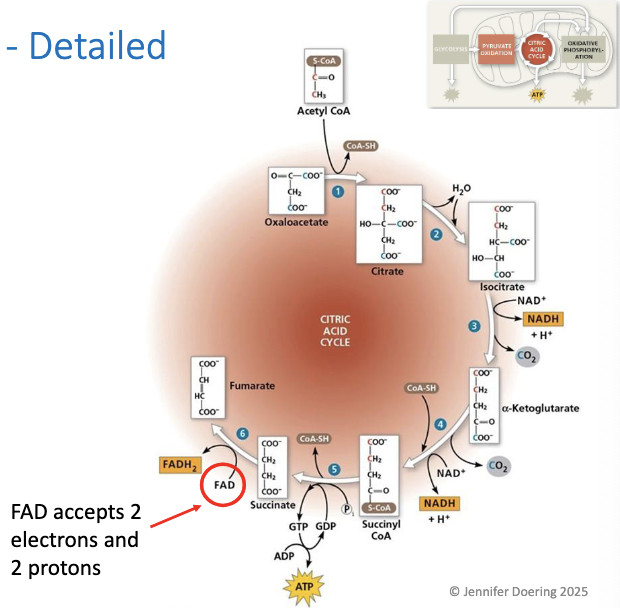
Two hydrogens are transferred to FAD, forming FADH2 and oxidizing succinate to result ini fumarate
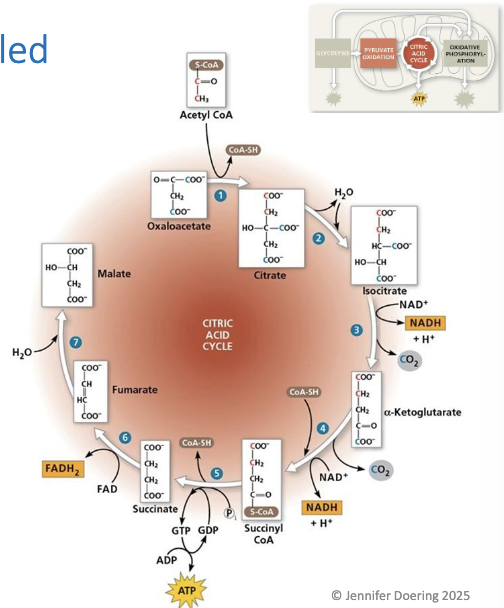
Addition of water rearrangess the bonds, turning fumarate inito malate
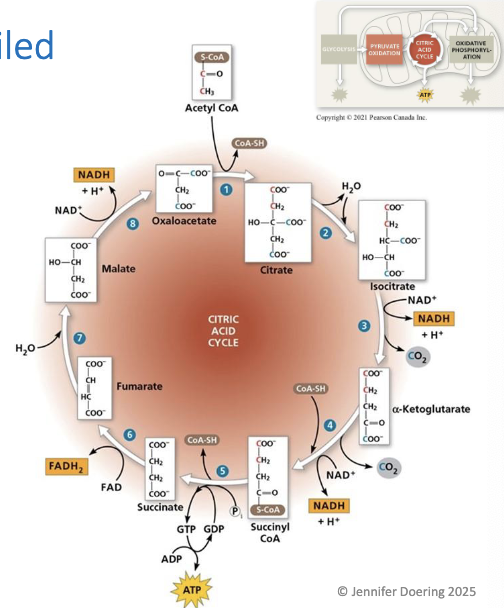
Malate is oxidized, reducing NAD+ to NADH, which forms oxaloacetate
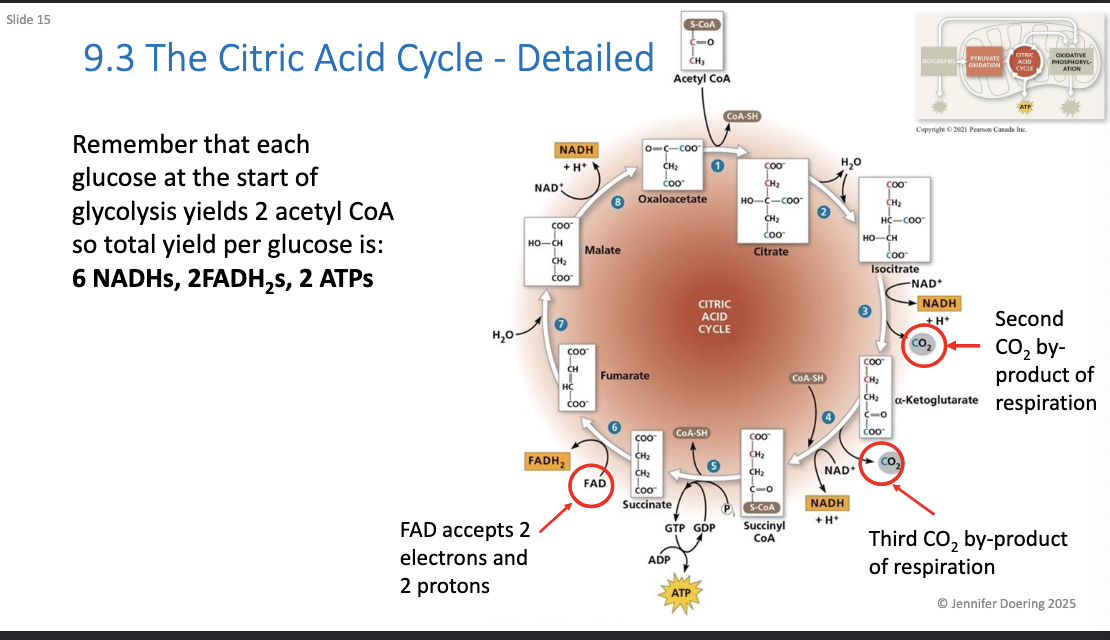
What has yielded from glycolysis and citric acid?
4 ATP per glucose molecule via SLP
2 ATP from glycolysis
2 ATP from citric acid cycle
Electron transport chain
a collection of protein complexes within the inner membrane of the mitochondrion
each component becomes reduced when it accepts e- from its “uphill” neighbour since its EN is less uphill
the transporters then return to the oxidized state and returns to the cycles to pick up more electrons
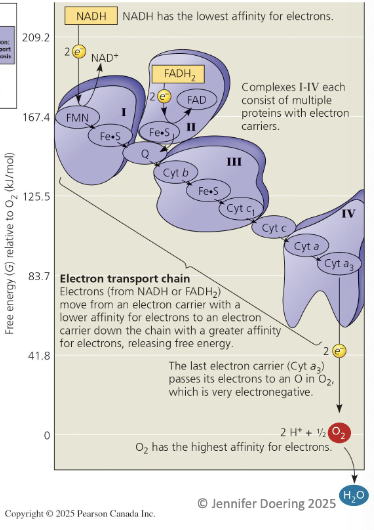
What happens to electrons from NADH in the electron transport chain?
Electrons from glycolysis and the citric acid cycle (via NADH) are transferred to the ETC complex I.
The first molecule in complex I is a flavoprotein (FMN), which gets reduced as NADH gives up its electrons.
FMN passes electrons to iron-sulfur protein (Fe-S) in complex I and returns to its oxidized form.
Electrons then move to ubiquinone (Q/coenzyme Q).
Next, electrons are transferred to cytochromes, which contain a heme (iron) group that accepts/donates electrons.
Complexes III and IV both have cytochromes.
Cytochromes pass electrons to molecular oxygen (O₂), which picks up 2 electrons + 2 protons to form water (H₂O).
How do electrons from FADH₂ enter the electron transport chain?
Electrons from the citric acid cycle (via FADH₂) enter through complex II.
Complex II is at a lower energy level than complex I and NADH.
Both NADH and FADH₂ donate the same number of electrons for oxygen reduction, but FADH₂ generates about 1/3 less energy than NADH.
ATP Synthase
a protein complex that is found throughout the inner membrane of the mitochondrion
makes ATP from ADP + inorganic P
oxidative phosphorylation
Works like an ion pump but in reverse
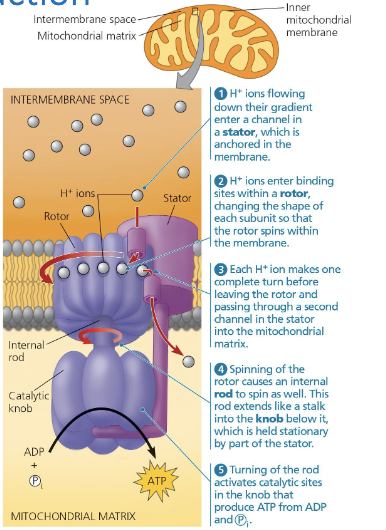
How does ATP synthase work
H+ ions flow down their concentration gradient to enter a channel called that stator
H+ ions ions bind with their sites ini the rotor, changing the shape of the protein subunit so that it spins (like a centrifuge)
Each H+ ion makes 1 complete turn before leaving the rotor and passing into a second channel in the stator and into the mitochondrial matrix
The spinning rotor causes a rod to spin ass well, which extends int oa knob (held stationary by the stator)
Turning of the rod activates catalytic sites that produce ATP from ADP in the knob
How does the ETC create the H+ gradient in mitochondria?
The exergonic flow of electrons in the ETC provides energy to pump H+ ions from the mitochondrial matrix to the intermembrane space (between the inner and outer mitochondrial membranes).
This creates a high concentration of H+ in the intermembrane space.
The stored gradient represents potential energy called the proton-motive force.
What happens to the H+ ions after they are pumped into the intermembrane space?
H+ ions diffuse back into the mitochondrial matrix through ATP synthase.
The flow of H+ drives the production of ATP from ADP + Pi.
What is chemiosmosis?
Chemiosmosis is an energy-coupling mechanism.
It uses energy stored in the form of an H+ gradient across a membrane to drive cellular work (such as ATP synthesis).
Cellular respiration: Accounting
What happens if there is no oxygen?
Anaerobic respiration: respiration in which the final e- acceptor is not oxygen
Anaerobic respiration is respiration in which the final electron acceptor is not oxygen.
It still uses an ETC, but the final electron acceptor is different depending on the species.
Example: some sulfate-reducing bacteria use SO₄²⁻ as the final electron acceptor.
How is fermentation different from anaerobic respiration?
Fermentation does not have an ETC or oxygen as the final electron acceptor.
It is not considered cellular respiration.
Its main role is to recycle NAD⁺ from NADH during glycolysis.
Why is NAD⁺ recycling important for ATP production?
Glycolysis produces ATP via substrate-level phosphorylation and needs a supply of NAD⁺ to carry electrons.
Without oxygen, NAD⁺ cannot be regenerated through the ETC, and the cell would run out of NAD⁺ (since it’s all reduced to NADH), preventing ATP production.
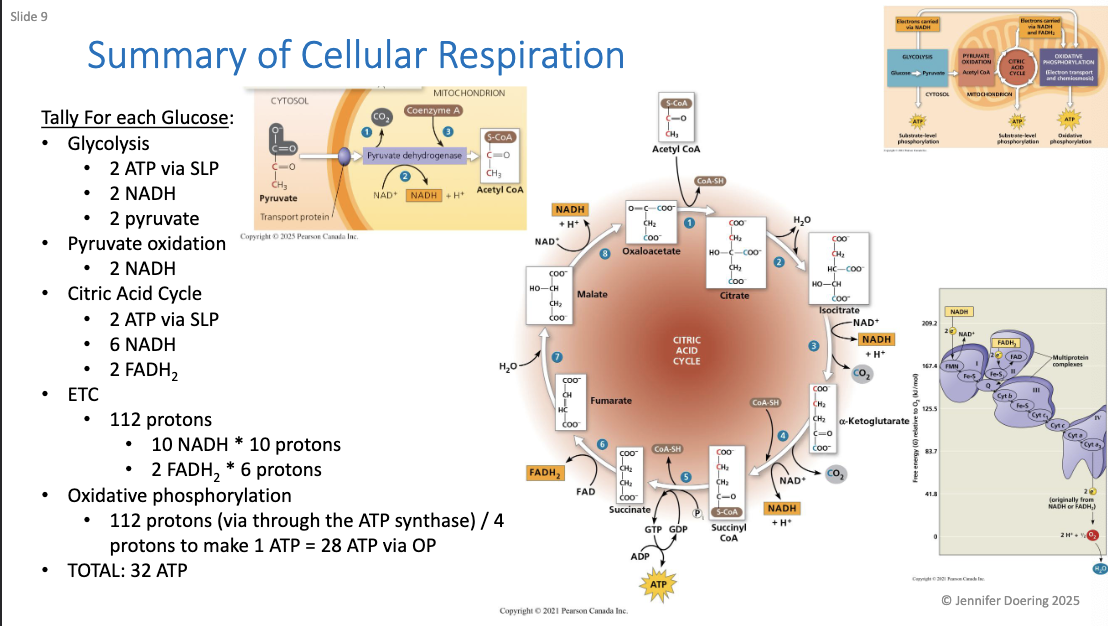
What happens during alcohol fermentation?
Pyruvate is converted to ethanol (ethyl alcohol).
CO₂ is released from pyruvate, forming 2 molecules of acetaldehyde.
Acetaldehyde is reduced by NADH to form ethanol.
This regenerates NAD⁺ needed for glycolysis.
Carried out by many bacteria and yeasts (e.g., sourdough, beer).
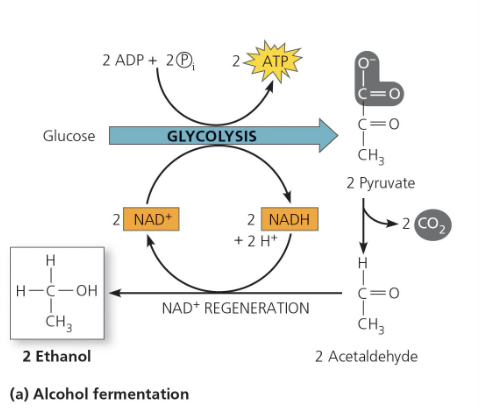
What happens during lactic acid fermentation?
Pyruvate is reduced directly by NADH to form lactic acid.
No CO₂ is released.
Carried out by many fungi and bacteria (used for cheese and yogurt).
In muscle cells during intense exercise (low oxygen), lactic acid fermentation occurs.
Blood carries lactate to the liver, where it is converted back to pyruvate and can re-enter the citric acid cycle when oxygen is available.
What do all ATP-generating processes (cellular respiration and fermentation) have in common?
All three use glycolysis to oxidize glucose to pyruvate (net 2 ATP).
All use NAD⁺ as the oxidizing agent.
How do fermentation and cellular respiration differ?
Fermentation:
Uses an organic molecule as the final electron acceptor to oxidize NADH.
Pyruvate in lactic acid fermentation
Acetaldehyde in alcohol fermentation
Yields 2 ATP via substrate-level phosphorylation (SLP).
Cellular respiration:
Uses the ETC to regenerate NAD⁺.
Yields up to 32 ATP via SLP + oxidative phosphorylation (OP).
What are obligate and facultative anaerobes?
Obligate anaerobes: can only perform fermentation or anaerobic respiration.
Facultative anaerobes: can switch between fermentation and cellular respiration depending on oxygen availability.
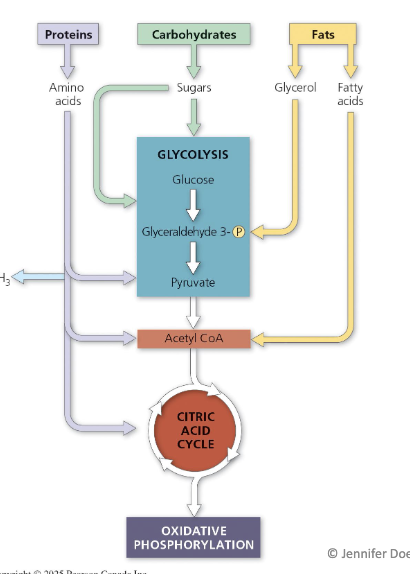
How do molecules from food connect to catabolic reactions?
Proteins: provide coenzymes, enzymes, and amino functional groups.
Carbohydrates: act as sugar sources for glycolysis.
Fats: broken down into glycerol (enters as G3P) and fatty acids (converted to Acetyl-CoA).
Fatty acids are broken down through beta oxidation.