1.3 Ions and the Octet Rule
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
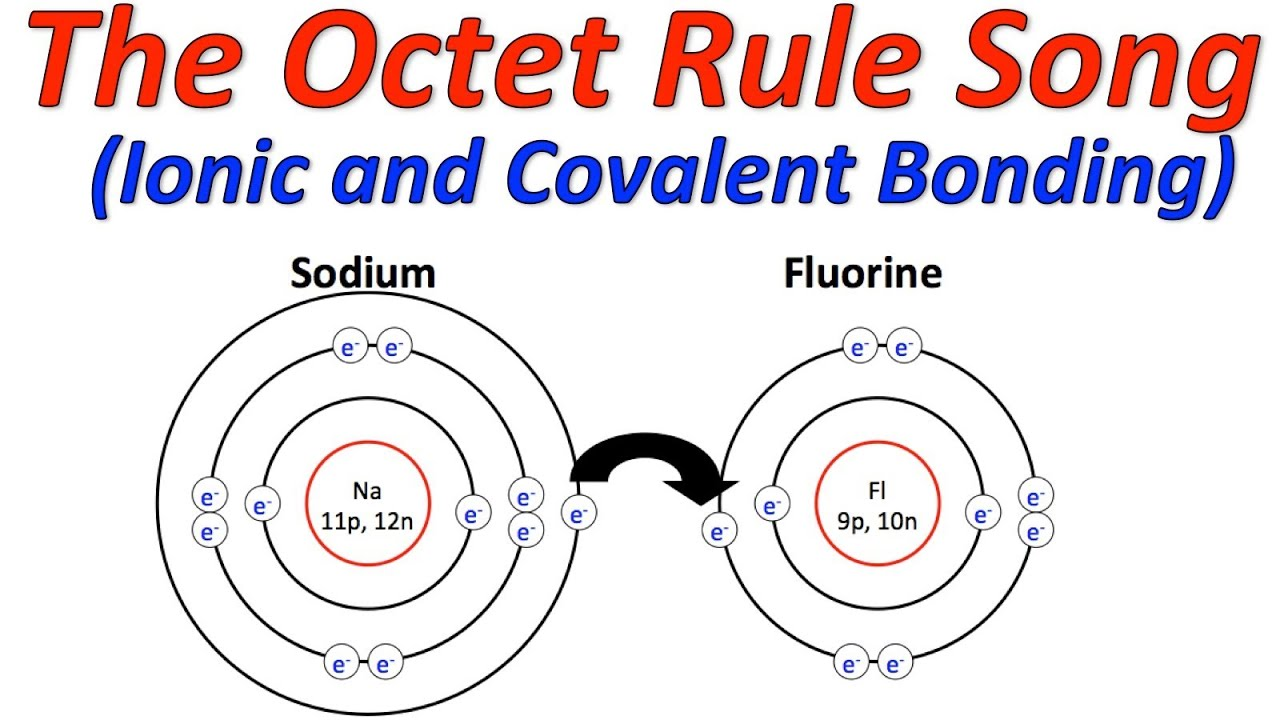
What is the Octet Rule
Full valence shells have a special stability
Full or stable octet - an electron arrangement where the valence shell is filled with 8 valence electrons (exception is He=2)
When atoms combine, they tend to attain this electron arrangemet.
This is known as octet rule.
What are three ways to get this done
Share electrons
Gain electrons
Lose electrons
Formation of Ions
Electron Arrangement
Netral atoms
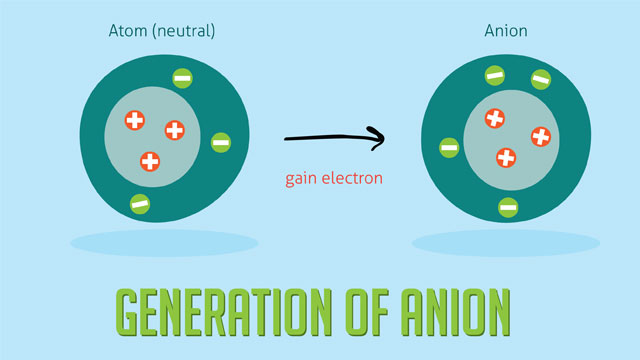
Explain the formation of Ions that are negative, also explain the name?
Anion
Formed by the acceptance of one or more shell of a neutral atom.
Carries a negative charge.
Electron Arrangement
Netral atoms
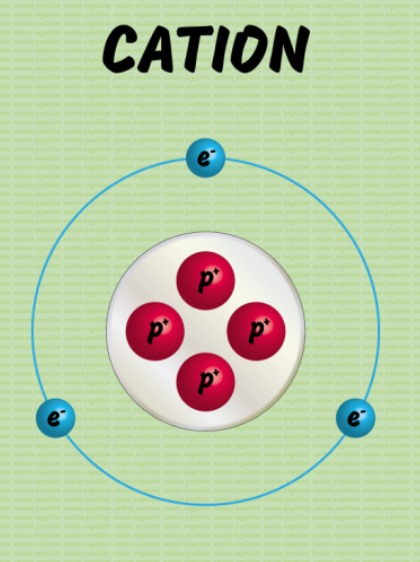
Explain the formation of Ions that are positive, also explain the name?
Cations
Formed by the removal of one or more electron from the valence shell of a neutral atom
Carries a positive charge
Explain Electron Arrangement Ions
Multivalent metals
Multivalent metals are metals with more than one ion charge. These elements can form different ions depending on the chemical reaction they undergo.
Explain Electron Arrangement Ions
Polyatomic ions
Polyatomic Ions are ions composed of more than one atom.
Polyatomic ions have distinct names.
1+ Charge
ammonium NH4+
3- Charge
Phosphate PO43-
Phosphite PO33-
2- Charge
Carbonate CO32-
Sulfate SO42-
Sulfite SO32-
Peroxide O22-
1- Charge
Hydrogen carbonate (bicarbonate) HCO3-
Hydroxide OH-
Nitrate NO3-
Nitrite NO2-
Chlorate ClO3-
what are they called
Common Polyatomic ions