Week 2: Amino Acids, Peptides, Protein Structure, & Collagen
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
What are the functional classes of proteins?
Enzymes (catalysis), transport, structure, movement, signaling, defense
What do all amino acids have?
Central alpha-carbon, amino group (-NH3+), carboxyl group (-COO^-), R group
What are the key properties of amino acids?
Chirality: the alpha-carbon is a chiral center for 19/20 amino acids (except for Glycine)
Stereochemistry: built exclusively from L-isomers of amino acids
Zwitterions: at physiological pH 7.4, they have both positive and negative charges
What are the nonpolar, aliphatic R groups?
Glycine, Alanine, Valine, Leucine, Isoleucine, Proline
Property: hydrophobic (oily)
Function: cluster in the protein interior, away from water; a major driver of protein folding
Glycine
Nonpolar, aliphatic; Gly [G]
![<p>Nonpolar, aliphatic; Gly [G]</p>](https://knowt-user-attachments.s3.amazonaws.com/76343043-8788-45ad-b7a2-d19a369c1d2d.jpg)
Alanine
Nonpolar, aliphatic; Ala [A]
![<p>Nonpolar, aliphatic; Ala [A]</p>](https://knowt-user-attachments.s3.amazonaws.com/640925de-d6b1-4776-93c4-83817b093ebd.png)
Valine
Nonpolar, aliphatic; Val [V]
![<p>Nonpolar, aliphatic; Val [V]</p>](https://knowt-user-attachments.s3.amazonaws.com/12909d9a-c78c-446e-816d-f69bc5f23061.png)
Leucine
Nonpolar, aliphatic; Leu [L]
![<p>Nonpolar, aliphatic; Leu [L]</p>](https://knowt-user-attachments.s3.amazonaws.com/d8306e46-1d64-43aa-812a-0064a576801a.jpg)
Isoleucine
Nonpolar, aliphatic; Ile (T)
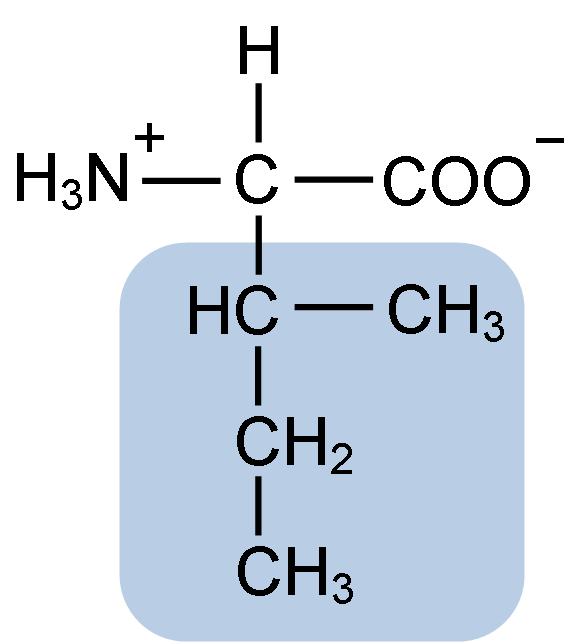
What are the aromatic R groups?
Phenylalanine, Tyrosine, Tryptophan
Property: generally hydrophobic; absorbs UV light
Tyrosine and tryptophan have partial polarity, making them less hydrophobic than phenylalanine
Phenylalanine
Aromatic; Phe [F]
![<p>Aromatic; Phe [F]</p>](https://knowt-user-attachments.s3.amazonaws.com/4b61fff0-80ef-4236-a22c-7e46f073df41.png)
Tyrosine
Aromatic; Try [Y]; anti-cancer drugs inhibit tyrosine binding
![<p>Aromatic; Try [Y]; anti-cancer drugs inhibit tyrosine binding</p>](https://knowt-user-attachments.s3.amazonaws.com/62b7fe4e-5577-4796-a889-fcad49425036.png)
Tryptophan
Aromatic; Trp [W]; essential amino acid to take in food
![<p>Aromatic; Trp [W]; essential amino acid to take in food</p>](https://knowt-user-attachments.s3.amazonaws.com/5fcdabe8-bb96-4eaa-9b53-edf5d6ac1a62.png)
What are the polar, uncharged R groups?
Serine, Threonine, Cysteine, Asparagine, Glutamine
Property: hydrophilic; can form hydrogen bonds
Location: found on protein surface
Serine
Polar, uncharged; Ser [S]
![<p>Polar, uncharged; Ser [S]</p>](https://knowt-user-attachments.s3.amazonaws.com/dd48ef04-0402-4b05-a138-40044bf9f3cc.png)
Threonine
Polar, uncharged; Thr [T]
![<p>Polar, uncharged; Thr [T]</p>](https://knowt-user-attachments.s3.amazonaws.com/102a643c-ad41-4c0b-845a-6ffe8d64852a.png)
Proline
Pro [P]; rigid, ring structure; creates kinks or bends and is known as a "helix breaker"
![<p>Pro [P]; rigid, ring structure; creates kinks or bends and is known as a "helix breaker"</p>](https://knowt-user-attachments.s3.amazonaws.com/49505532-6847-42b7-81a5-7d83d70f8257.png)
Cysteine
Cys [C]; contains a sulfhydryl (-SH) group that can form a covalent disulfide bond
![<p>Cys [C]; contains a sulfhydryl (-SH) group that can form a covalent disulfide bond</p>](https://knowt-user-attachments.s3.amazonaws.com/93395ec8-88e4-4ef5-8ed0-d3d74b216ddf.png)
Histidine
His [H]; pKa near physiologic pH, allowing it to act as a proton donor/acceptor in enzyme catalysis
![<p>His [H]; pKa near physiologic pH, allowing it to act as a proton donor/acceptor in enzyme catalysis</p>](https://knowt-user-attachments.s3.amazonaws.com/08bc00ea-4c29-4379-97d8-3c7f91aa1e4e.png)
What are negatively charged (acidic) groups?
Aspartate, Glutamate
Property: very hydrophilic; deprotonated (negative) at pH 7.4
Aspartic Acid (Aspartate)
Negatively charged; Asp [D]
![<p>Negatively charged; Asp [D]</p>](https://knowt-user-attachments.s3.amazonaws.com/6743e018-998f-4d0a-b74c-4fb977dc0855.jpg)
Glutamic Acid (Glutamate)
Negatively charged; Glu [E]
![<p>Negatively charged; Glu [E]</p>](https://knowt-user-attachments.s3.amazonaws.com/156445ee-c656-47f3-80b4-3b1726c403e8.png)
What are the positively charged (basic) groups?
Lysine, Arginine, Histidine
Property: very hydrophilic; protonated (positive) at pH 7.4 (except Histidine can be neutral)
Lysine
Positively charged; Lys [K]
![<p>Positively charged; Lys [K]</p>](https://knowt-user-attachments.s3.amazonaws.com/99ff7950-ea78-400d-b6d5-155174694639.png)
Argine
Positively charged; Arg [R]
![<p>Positively charged; Arg [R]</p>](https://knowt-user-attachments.s3.amazonaws.com/a1dc8e4c-fef8-42cf-9926-207c25919de5.png)
Isoelectric point (pl)
The specific pH at which an amino acid has a net charge of zero; low pH -> positive charge; high pH -> negative charge
Peptide bond
Links amino acids together with the amide bond being formed via a dehydration reaction; rigid and planar due to partial double-bond character (no rotation); forms a repeating backbone sequence; R groups project outwards from the backbone; ALWAYS FORMED B/T AMINO GROUP AND CARBOXYLIC GROUP
N-Terminus
At the end where the amino group is (left side)
C-Terminus
At the end where the carboxylic group is (right side)
What is Glutathione (GSH)?
Tripeptide composed of glutamate, cysteine, and glycine (Glu-Cys-Gly); a major antioxidant in cells, protecting them from oxidative damage
Insulin and glucagon
Regulate blood glucose
Vasopressin and oxytocin
Small peptide hormones with critical physiological roles
What is the hierarchy of protein structure?
Primary, secondary, tertiary, quaternary
Primary structure
A linear sequence of amino acids determined by DNA and stabilized by covalent peptide bonds
Function: the "blueprint" that dictates all higher levels of folding
Ex: Sickle Cell
Secondary structure
Regular, repeating patterns of the polypeptide backbones and is stabilized by hydrogen bonds between backbone C=O and N-H groups; R groups aren't involved in stabilization
Alpha-helix
A rigid, right-handed spiral
H-bonds: between C=O of residue n and N-H residue n+4
R groups: project outwards from helix
Found in hair and myoglobin
Disrupted by: Proline, Glycine, bulky or charged R groups
Beta-sheet
Formed from two or more polypeptide segments lined up side-by-side and backbone is in an extended, zig-zag conformation
H-bonds: form b/t adjacent strands
Types: antiparallel and parallel
R groups: project alternatively above and below the sheet
Tertiary structure
Overall 3D shape of a single polypeptide chain and describes how secondary structures are packed together; stabilized by interactions b/t R groups
What are the interactions that stabilize the tertiary structures?
Hydrophobic (primary driving force): nonpolar R groups buried in protein core
Hydrogen: b/t polar R groups
Ionic bonds (salt bridges): b/t oppositely charged R groups
Disulfide: covalent bond b/t two Cysteine residues; acts as a "molecular staple"
Quaternary structure
Applies only to proteins w/ more than one polypeptide subunit and describes the arrangement and interaction of subunits; held together by the same forces as tertiary
What is protein folding?
A polypeptide chain acquires its native 3D structure and is driven by the hydrophobic effect and assisted by chaperone proteins (prevent misfolding and aggregation)
What is misfolding?
Can lead to loss of function or creation of a toxin protein causing disease
What is denaturation?
Loss of a protein's secondary, tertiary, and quaternary structures resulting in loss of biological function
What are denaturing agents?
Heat (disrupts weak interactions), extreme pH (alters charge on R groups, disrupts ionic bonds), detergents & urea (disrupts hydrophobic interactions)
What is collagen?
Most abundant protein in the body (25%); fibrous structural protein; provides tensile strength to connective tissues; found in skin, bones, tendons, cartilage, blood vessels
What is collagen's structure?
Gly-X-Y (X= Proline, Y= Hydroxyproline); triple helix; Glycine's small size allows tight packing of chains
What is the post-translational modification of collagen?
Hydroxyproline and hydroxylysine are created after translation. Prolyl hydroxylase and lysyl hydroxylase add -OH groups and they are essential for hydrogen bonds that stablize the triple helix
Vitamin C (Ascorbic Aids)
Required cofactor for prolyl and lysyl hydroxylase; keeps iron in the enzyme's active site in reduced (Fe2+) state which is necessary for functioning; deficiency directly impairs synthesis of stable collagen
Scurvy
Cause: dietary deficiency of Vitamin C
Biochemical defect: inactive prolyl and lysyl hydroxylases
Pathophysiology: collagen chains are synthesized but not hydroxylated; a stable triple helix can’t be formed; connective tissue becomes weak and fragile
Symptoms: easy brusiing, bleeding gums, poor wound healing
Osteogenesis Imperfecta (OI)
“Brittle Bone Disease”
Cause: genetic mutation in Type I collagen genes
Biochemcial defect: often, a substitution of a bulky amino acid for a critical Glycine
Pathophysiology: the bulky R group distrupts the tight packing of the triple helix, creating weak collagen
Symptoms: brittle bones, fractures, blue sclerae
Ehlers-Danlos Syndrome
Cause: group of genetic disorders
Defect: often involves defective corss-linking of collagen fibrils
Symptoms: hypermobile joints, fragile skin