IMED1002 - Proteins (Amino Acids, Peptides, Structure, Function)
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
Amino Acids you need to be able to recognise and draw
- Ala
- Gly
- Phe
- Tyr
Peptides
- there are two rotatable backbone bonds per residue
Quarternary Structure
- proteins that contain more then one chain (subunit) (arrangement of subunits and their contacts)
- e.g haemoglobin has 4 subunits, 2 alpha and 2 beta pleated sheet subunits
Tertiary Structure
- folding and bonding of AA that are further apart to form 3D structure.
- final folded form of many proteins interfolding of Beta pleated sheets and alpha helices
Protein Domains (NOT NEEDED TO KNOW)
- chains of 200+ AA often fold into several compact stable structures known as domains
- these 3D structures in proteins usually have a specific function, such as binding of small molecules
- many domains are structurally independent, with characteristics of small proteins
Transmembrane Proteins
- Alpha helical regions
- membranes are phospholipid bilayers with embedded proteins
Basic side chains
lysine, arginine, histidine
acidic side chains
aspartic acid, glutamic acid
uncharged polar side chains
asparagine, glutamine, serine, threonine, tyrosine
side chains that have electronegative atoms
serine, threonine, tyrosine
Simple Protein
protein consisting of only AA and no other chemical groups
Conjugated Protein
proteins that contain other chemical components in addition to AA (e.g Hb)
Prosthetic Group
non-protein component of a conjugated protein.
Conjugated Proteins
Classification: Chemical nature of the prosthetic group
- Lipoproteins: contains lipids
- Metalloproteins: contain a specific metal
- Glycoproteins: contain sugar groups
Amino Acid Chirality
- all amino acids are chiral except glycine
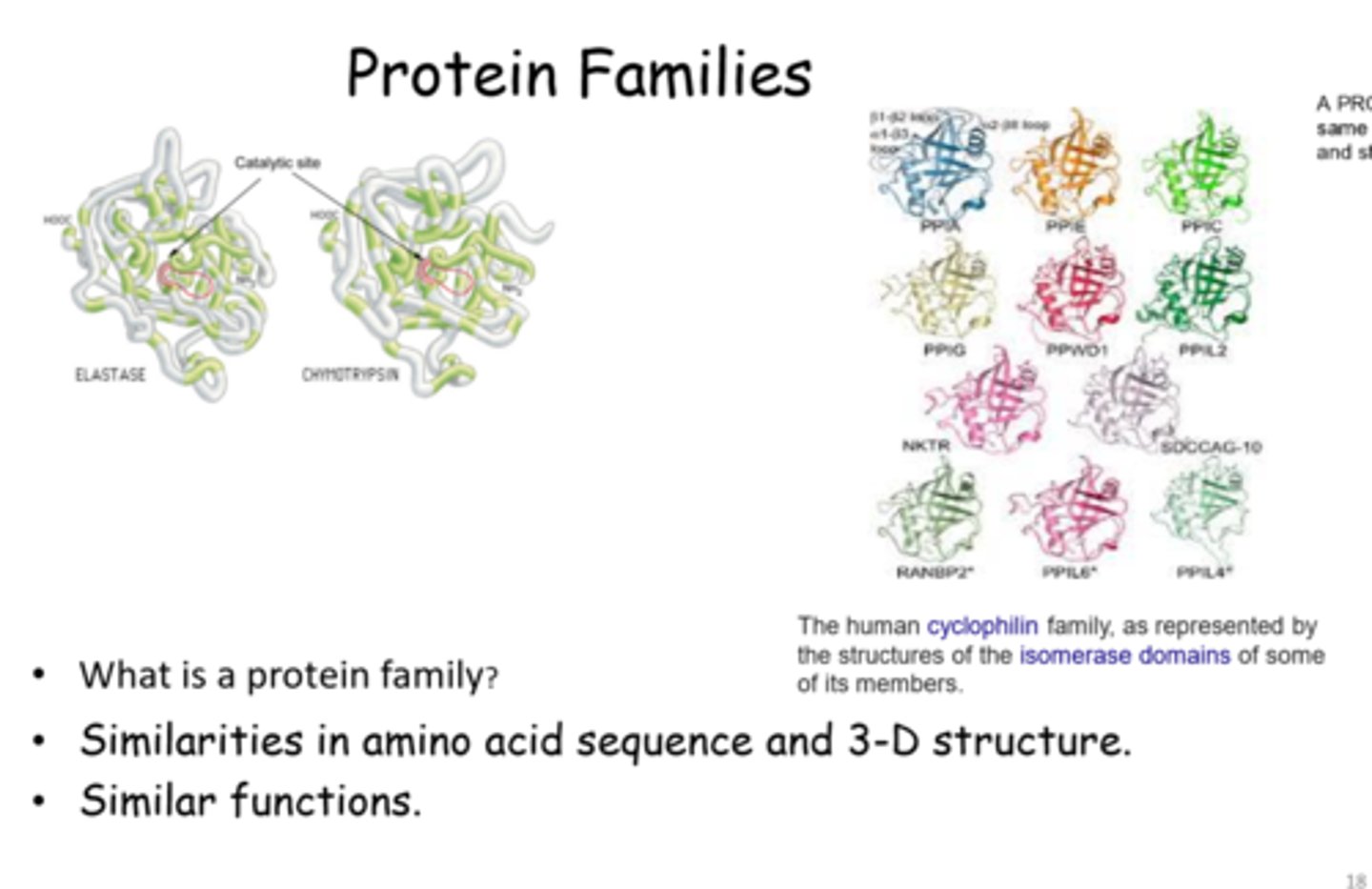
Protein Family
A group of polypeptides that shares a similar amino acid sequence or three-dimensional structure, reflecting a common evolutionary origin. Individual members often have related but distinct functions, such as kinases that phosphorylate different target proteins.
Native Protein
a protein with the shape (secondary, tertiary, and quaternary structure) in which it exists naturally in living organisms so that it can fold
Things that cause protein denaturation
- heat breaks H-bonds and thus denatures proteins
- chemicals can denature proteins
- acids, bases and salts
- detergents
- heavy metals (these react with -SH groups)
Denaturation
- When a native protein is denatured it nearly always loses its biological activity (as denaturation changes secondary, tertiary and quarternary structures)
- Denaturation does not alter the primary structure
Protein Functions
- Transport proteins: carry things around e.g Hb
- Storage proteins: ferritin - Fe, ovalbumin - egg (includes nutrient proteins)
- Protective Proteins: defend against infection and injury
- Movement proteins: (motile proteins) cells and organisms e.g actin, myosin
- Regulatory proteins: control cellular and physiological activity, signalling and receptors e.g hormones - insulin, cytokines and growth factors
- Structural Proteins: support cell and organ shape e.g collagen - ECM (extracellular matrix), ligaments, elastin, hair, nails
- Other Proteins: not so easy to classify as have exotic functions e.g anti freeze proteins. elastic proteins
Fibrous Protein
A protein that has only a secondary structure; generally insoluble; includes collagens, elastins, and keratins. (usually structural)
Globular protein
Any protein in which the polypeptide chain folds into a compact, rounded shape. Includes most enzymes. (usually functional)
- has hydrophobic core region contains non-polar side chains, with polar side chains on the outside fo the molecules (which can form hydrogen bonds with water)
Ligand
A molecule that binds specifically to another molecule, usually a larger one.
- e.g ions, small molecules, macromolecules. Ligands bind to molecules in specific ways, and multiple ligands can bind simultaneously
Antibodies (Immunoglobins)
- bind antigens - many different types, so have a huge diversity of Immunogloblins, due to changes in AA sequence, and thus structure of protein at antigen binding site. The antibody binds a specific antigen which is a ligand.
Enzymes
- most enzymes are globular proteins
- enzyme bind specific ligand (substrate), alter its configuration so that it is more easily changed into the product while the enzyme itself is unchanged
- Substrate binding involves non covalent interactions in the enzymes active site
- organisation of atoms in the active site is optimised for substrate binding and catalysts.
Properties of Enzymes
- enzymes do not change equilibrium of a reaction, so if a reaction cant take place an enzyme wont make it happen, it will just increase the reaction rate
- metabolic reactions - conversion of one compound (substrate, the reactant) into another compound (the products)
- enzyme catalysed reactions are usually linked in series or arrays to form metabolic pathways
- often the product of one reaction can influence another reaction in the sequence, this forms a metabolic pathway
Altering Enzyme Activity
- enzyme activity needs to be well controlled (regulated) to ensure proper reactions occur in cells. This often involves binding the enzyme. Some enzymes are regulated by phosphorylation. Adding or removing P groups changes the shape and thus activity of the enzyme
Naming Enzymes
- Biological catalysts that speed up the rate of reaction without becoming part of the reaction (i.e enzyme is not permenantly changed)
- Enzyme name: typically reflects first the substrate and then clarifies reaction catalysed (ends in "ase")
- e.g lactate dehydrogenase has lactate as its substrate and dehydrogenates (removes H from) the substrate
- some names dont fit in this e.g trypsin
Enzymes and Redox reactions
- dehydrogenases and oxidases
- can involve simply electron transfer or can involve transfer of H, i.e proton and electrons
(Enzyme Class) ligase
catalyse formation of bonds (joining)
(Enzyme Class) polymerase
catalyse polymerisation reactions (e.g DNA and RNA synthesis)
(Enzyme Class) kinase
catalyse addition of phosphate groups to molecules
(Enzyme Class) phosphatase
catalyse hydrolytic removal of a phosphate group from a molecule
(Enzyme Class) protease
break (hydrolyse) peptide bonds in proteins
(Enzyme Class) oxidoreductase
catalyses oxidation/reduction