Basic Chemistry
0.0(0)
Card Sorting
1/62
Earn XP
Description and Tags
Last updated 11:34 AM on 9/9/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
63 Terms
1
New cards
Element
Atoms with the same number of protons (atomic number); can’t be broken down by chemical reactions
2
New cards
Compound
2 or more elements that are in the same molecule or a salt
3
New cards
4 most abundant elements found in an organism’s body
Carbon, Hydrogen, Oxygen, and Nitrogen (CHON)
4
New cards
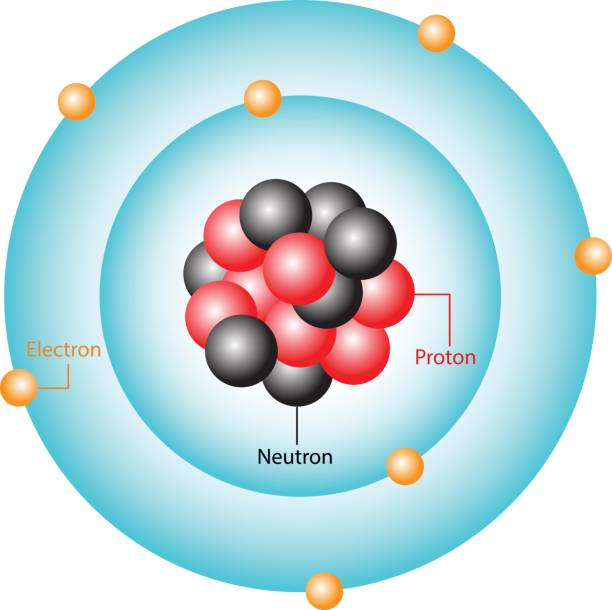
Proton (charge, location, mass)
subatomic particle with a positive charge; located in the nucleus; 1 amu
5
New cards
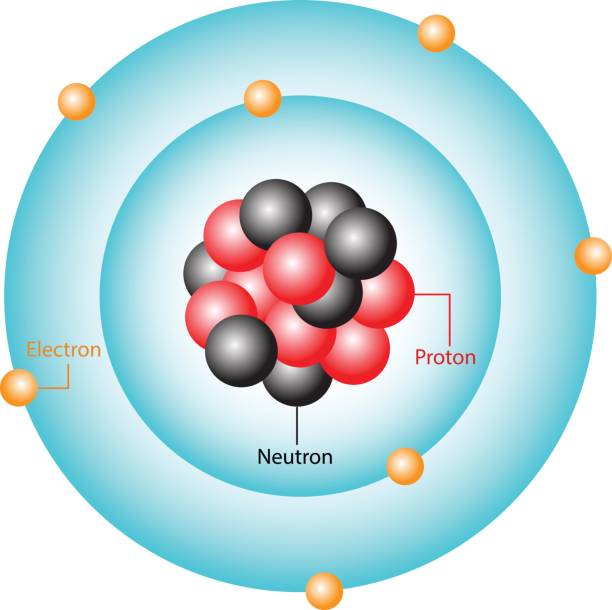
Electron (charge, location, mass)
subatomic particle with a negative charge; located outside of the nucleus in electron clouds; 0 amu
6
New cards
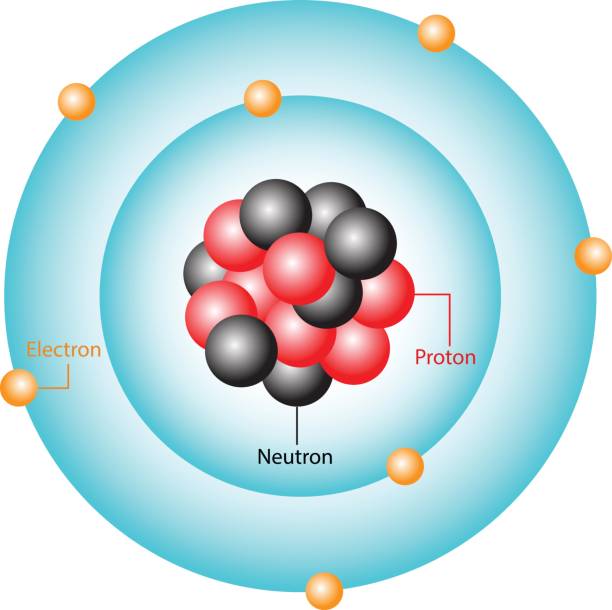
Neutron (charge, location, mass)
subatomic particle with neutral charge; located in the nucleus; 1 amu
7
New cards
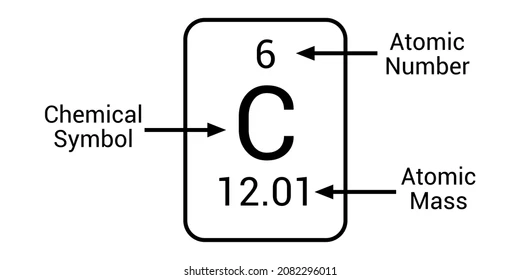
Atomic Number
Number of Protons; Determines what element an atom is
8
New cards
Mass Number
# of protons + # neutrons
9
New cards
How do you use the Atomic Number & the Mass Number to determine the number of neutrons in an atom?
# of Neutrons = Mass Number - Atomic Number
10
New cards
Isotope
Atoms of the same element that differ in the number of neutrons
11
New cards
How are radioactive isotopes used in biology?
Used for Carbon-dating fossils and as Metabolic Tracers (tracing chemical reactions in organisms)
12
New cards
How many electrons can occupy each shell / energy level in an atom?
1st Shell: 2 electrons
2nd Shell: 8 electrons
3rd Shell: 8 electrons
2nd Shell: 8 electrons
3rd Shell: 8 electrons
13
New cards
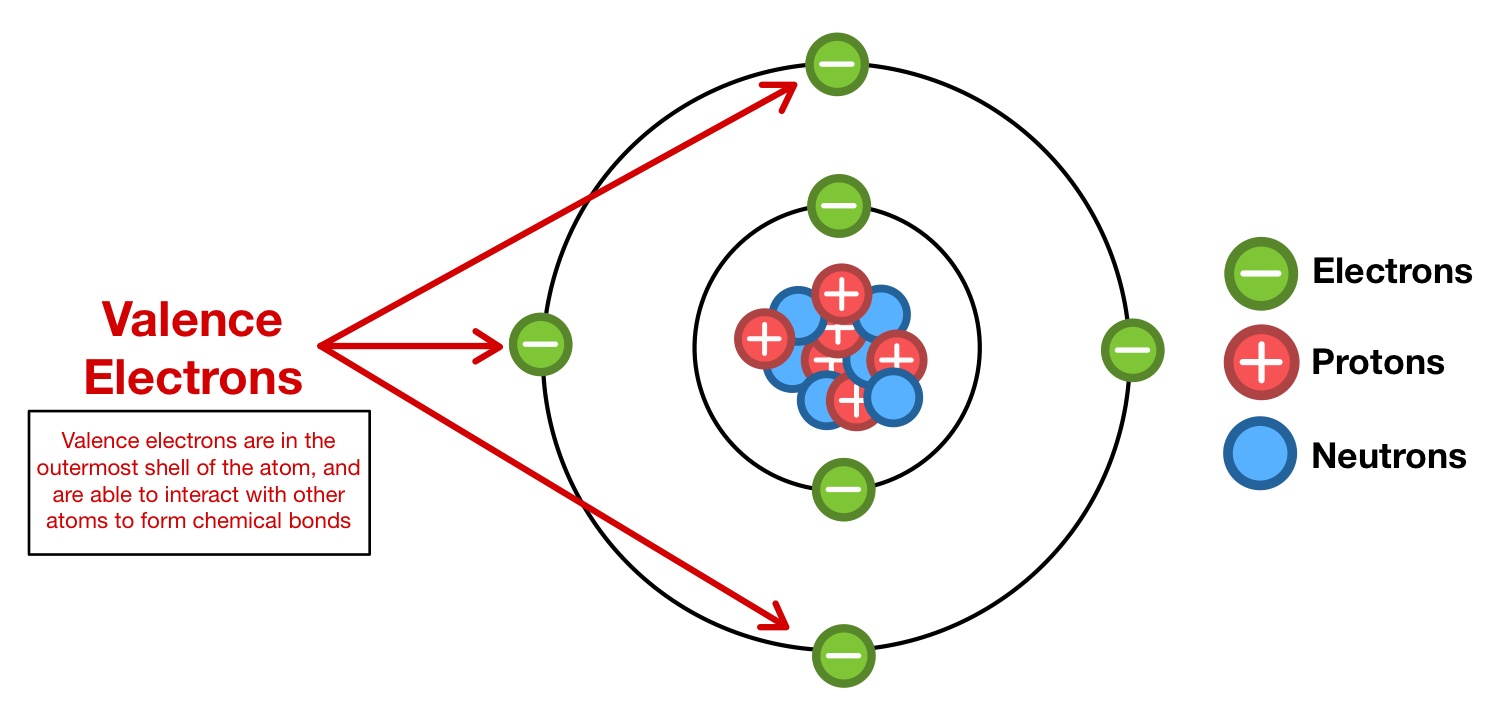
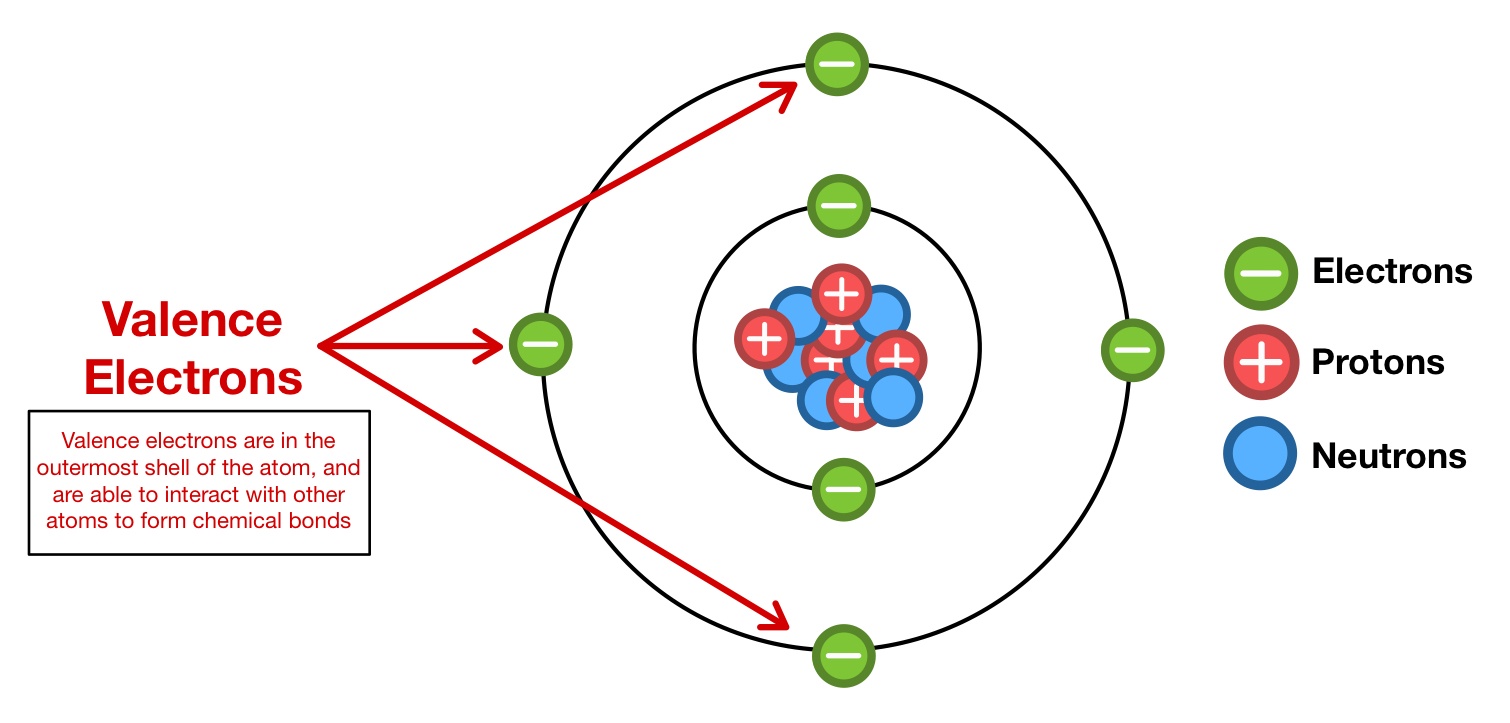
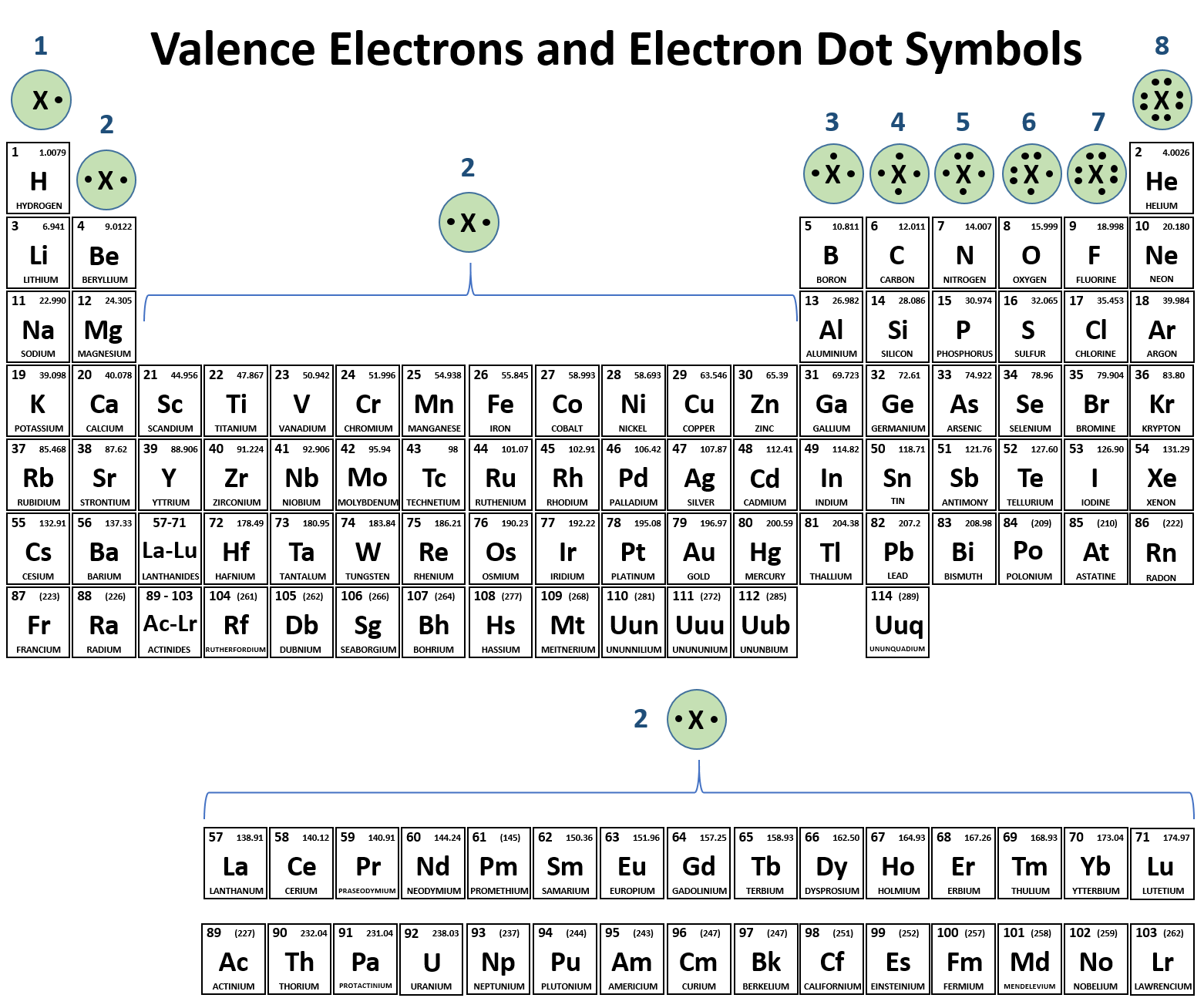
Valence Shell
Outermost shell that contains the valence electrons
14
New cards
Valence Electrons
Electrons in the outermost shell (Valence shell)
15
New cards
Valence
the number of electrons an atom needs to fill the valence shell
16
New cards
What determines the chemical behavior of an element?
The Valence Number (# of unpaired electrons in the valence shell) determines an element's reactivity to other atoms
17
New cards
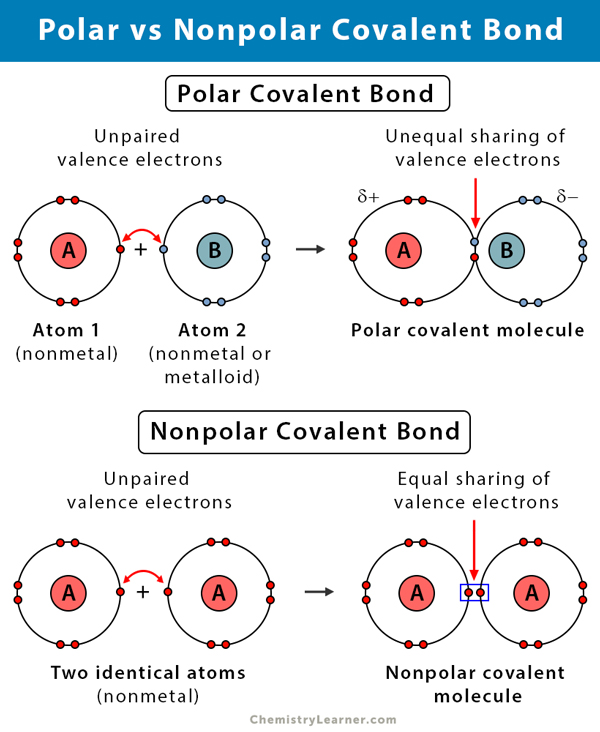
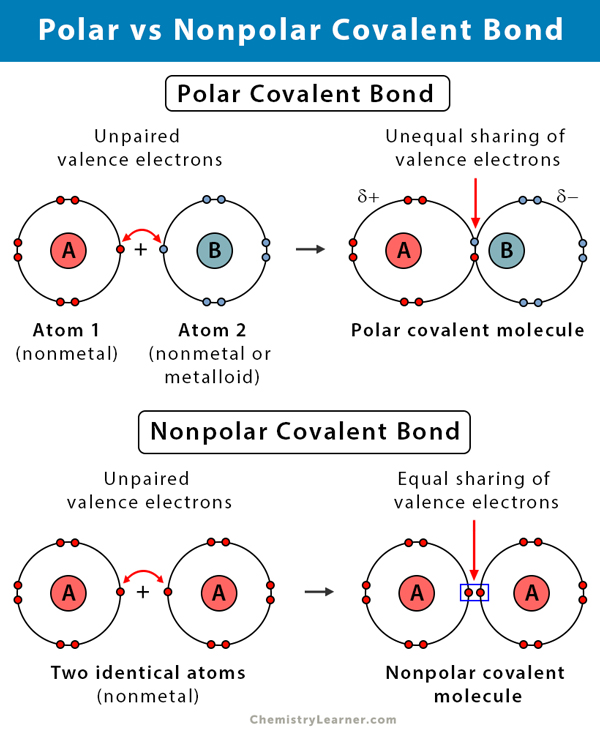
Polar Covalent Bond
Bond created through an unequal sharing of electrons; creates a charge
18
New cards
Non-polar Covalent Bond
Bond created through the equal sharing of electrons; does not create a charge
19
New cards
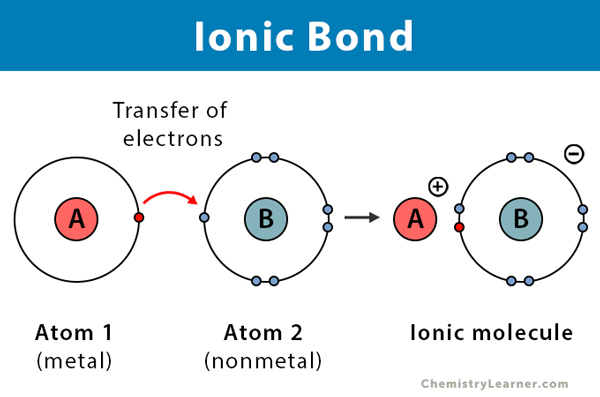
Ionic Bond
one atom donates electrons to another atom because of extreme differences in electronegativity
20
New cards
Hydrogen Bond
caused by partial charges; partial negative of a molecule is attracted to partial positive of Hydrogen in another molecule
21
New cards
Van Der Waals
-Because of the random motion of electrons, at times, electrons may accumulate on one side of the molecule, creating “hotspots” of negative and positive charge
-When molecules are very close together, many weak Van Der Waals interactions may result in enough force to alter the shape of large molecules as the molecules within proximity of each other cling to one another
-When molecules are very close together, many weak Van Der Waals interactions may result in enough force to alter the shape of large molecules as the molecules within proximity of each other cling to one another
22
New cards
Know which two biologically important elements are most electronegative
Nitrogen and Oxygen
23
New cards
Which elements in the periodic table are most likely to form ionic bonds with each other?
Elements that have a valence which that together add up to 8 would be most likely to form ionic bonds with each other. For example, a Sodium with one valence electron would want to give its electrons away to an element such as Chlorine with 7 valence electrons, since Chlorine wants 1 more valence electron to reach 8 and become stable.
24
New cards
Chemical Equilibrium
When the rate of forward reaction is equal to the rate of the reverse reaction; Direction of reaction depends on concentration of products and reactants
25
New cards
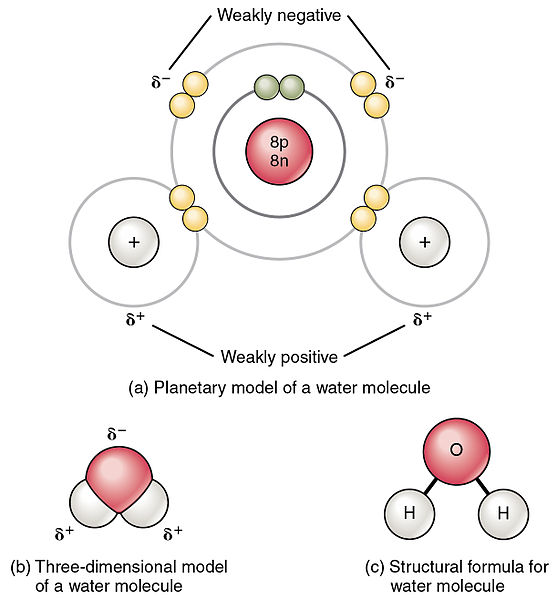
Explain why water is a polar molecule.
-Oxygen is more negative; Hydrogen is more positive
-There is a difference in electronegativity between atoms which causes unequal sharing of electrons in a molecule. Because of the partial charges within the molecule and the molecules shape, there are opposite poles: a positive hydrogen side and a negative oxygen side.
-Polar covalent means there is an unequal sharing of electrons, creating a charge.
-There is a difference in electronegativity between atoms which causes unequal sharing of electrons in a molecule. Because of the partial charges within the molecule and the molecules shape, there are opposite poles: a positive hydrogen side and a negative oxygen side.
-Polar covalent means there is an unequal sharing of electrons, creating a charge.
26
New cards
4 properties of water
Cohesive Behavior, Moderation of temperature (thermoregulation), expansion upon freezing, and versatile solvent/solvent of life
27
New cards
Cohesive Behavior of water
Water is cohesive because of its ability to form hydrogen bonds with other water molecules because water is polar; Significant because it allows for surface tension, transpiration, and photosynthesis
28
New cards
How does the cohesion of water help carry out transpiration? Significance?
-As a water molecule evaporates from the stomata in leaves, it pulls adjacent water molecules up the xylem against gravity (and then the next water molecule is pulled into the former's place).
-Transpiration allows for water to travel through the plant and the leaves, eventually leaving through the stomata
-Water carries nutrients and dissolves substances within the plant, allowing for photosynthesis and the continuation of the plant's life
-Transpiration allows for water to travel through the plant and the leaves, eventually leaving through the stomata
-Water carries nutrients and dissolves substances within the plant, allowing for photosynthesis and the continuation of the plant's life
29
New cards
How does the cohesion of water cause surface tension? Significance?
-Water has a very high surface tension (difficult for you to break the surface of the liquid). This is caused by Hydrogen Bonds between other water molecules on the surface and with the water molecules below, which is Cohesion
-Creates a habitat for life to exist on the surface of water.
-Creates a habitat for life to exist on the surface of water.
30
New cards
How does water's high specific heat help it moderate temperatures? Significance?
-Water has a high specific heat; Water can absorb and release large amounts of heat with minimal change in temperature.
-Large amounts of heat is absorbed during day/summer without changing temperature of water a lot
-Large amounts of heat released into air during night/winter without changing temperature a lot
-Moderates temperature of coastal climates; Stabilized ocean temperatures; Also stabilizes body temperature of organisms
-Large amounts of heat is absorbed during day/summer without changing temperature of water a lot
-Large amounts of heat released into air during night/winter without changing temperature a lot
-Moderates temperature of coastal climates; Stabilized ocean temperatures; Also stabilizes body temperature of organisms
31
New cards
Heat of Vaporization
the amount of heat needed (in Celcius) to change 1g of liquid into a gas
32
New cards
How does water's high heat of vaporization help it moderate temperatures? Significance?
-Water has a high heat of vaporization and absorbs a lot of heat before evaporating
-Allows for the moderation of earth’s climate
-Sun’s heat absorbed by tropical oceans causes the evaporation of surface water
-As moist air travels higher and poleward, it cools
-Hydrogen Bonds form, releasing heat along with the precipitation
-Allows for the moderation of earth’s climate
-Sun’s heat absorbed by tropical oceans causes the evaporation of surface water
-As moist air travels higher and poleward, it cools
-Hydrogen Bonds form, releasing heat along with the precipitation
33
New cards
How does water's evaporative cooling help it moderate temperatures? Significance?
-Water molecules with the most KE evaporate away, which leaves the average KE of remaining water molecules significantly lower (low temp)
-The evaporation of water from leaves of plants or skin of humans removes excess heat, which allows for organisms to thermoregulate
-The evaporation of water from leaves of plants or skin of humans removes excess heat, which allows for organisms to thermoregulate
34
New cards
Explain why water expands upon freezing
When water decreases in temp, it causes a decrease in KE, so Hydrogen bonds are stable and not constantly breaking. The water molecules then spread out creating a crystal lattice.
Because of the increase in volume, the density decreases and ice floats
Because of the increase in volume, the density decreases and ice floats
35
New cards
Significance of water expanding upon freezing
-Ice also releases heat when it forms and traps the heat in the underlying body of water.
-allows marine life to continue to exist under the ice
-protects plants covered in frost (orange groves in Florida)
-allows marine life to continue to exist under the ice
-protects plants covered in frost (orange groves in Florida)
36
New cards
Explain why water is a versatile solvent. Significance?
-Water dissolves substance with a charge because it is polar
-Water molecules for a hydration shell around positive and negative ions due to waters partial negative and positive charges
-Most chemical reactions needed to maintain life occur in aqueous solutions
-Water molecules for a hydration shell around positive and negative ions due to waters partial negative and positive charges
-Most chemical reactions needed to maintain life occur in aqueous solutions
37
New cards
Adhesion
attraction of molecules of one kind for molecules to a different kind
38
New cards
How does adhesion contribute to transpiration?
When stomata are closed the adhesion of water to walls of xylem (because the walls are polar) keeps the water from running back down to the trunk or stem
39
New cards
Cohesion
the attraction between the same kind of molecules
40
New cards
How does cohesion contribute to transpiration?
Water is cohesive because of it’s ability to form hydrogen bonds with other water molecules. As water evaporates through the stomata in leaves, it pulls adjacent water molecules up the xylem against gravity (the adjacent water molecules then taking the molecule which pulled it’s place)
41
New cards
Surface Tension
a measure of how difficult it is to break the surface of a liquid
42
New cards
Thermal Energy
kinetic energy associated with the TOTAL movement of atoms and molecules in a body of matter (Joule units)
43
New cards
Temperature
represents the AVERAGE kinetic energy of the atoms and molecules in a body of matter (Celcius units)
44
New cards
heat
thermal energy that’s transferred from one body of matter to another (Calorie units)
45
New cards
Specific heat
amount of heat gained or lost to change 1g of a substance 1 degree Celcius
46
New cards
Why is water's specific heat so high?
When heat is absorbed by water molecules, it first has to be used to break Hydrogen-bonds, before the average KE can be increased
When heat is lost from water and KE begins to decrease, Hydrogen bonds will start to form more often (because molecules aren’t moving around as much). The formation of hydrogen bonds release heat, increasing temp again.
When heat is lost from water and KE begins to decrease, Hydrogen bonds will start to form more often (because molecules aren’t moving around as much). The formation of hydrogen bonds release heat, increasing temp again.
47
New cards
Heat of vaporization
amount of heat a liquid needs to absorb to turn 1g of that liquid into gas
48
New cards
Why is water heat of vaporization so high?
Water has high heat of vaporization because water molecules need enough KE to break Hydrogen bonds before the water can leave the liquid and become a gas.
49
New cards
Why does the temperature of a liquid decrease during evaporation?
Evaporative cooling occurs because the water molecules with the MOST KE evaporate away, which leaves the average KE of remaining water molecules significantly lower, meaning the temperature has been reduced.
50
New cards
When H-bonds form is heat being released or absorbed?
Released
51
New cards
When H-bonds break is heat being released or absorbed?
Absorbed
52
New cards
Explain why ice floats and the significance to life on planet Earth.
-When water decreases in temperature, the Kinetic Energy decreases as well. So, the hydrogen-bonds are stable and not constantly breaking, meaning the water molecules spread out/held at an arms length away from each other (crystal lattice).
-Because of the increase in volume, the density decreases and the ice floats.
-It is significant for life because when ice forms, it releases heat and then traps that heat in the underlying body of water. This allows marine life to continue even if conditions outside of the water are too cold.
-Because of the increase in volume, the density decreases and the ice floats.
-It is significant for life because when ice forms, it releases heat and then traps that heat in the underlying body of water. This allows marine life to continue even if conditions outside of the water are too cold.
53
New cards
What is a hydration shell? What property of water is it associated with?
-layer of water molecules completely surrounding the ion
-It is associated with water being polar and contains both negative and positive charges, meaning that it is able to attract both negative and positive ions.
-It is associated with water being polar and contains both negative and positive charges, meaning that it is able to attract both negative and positive ions.
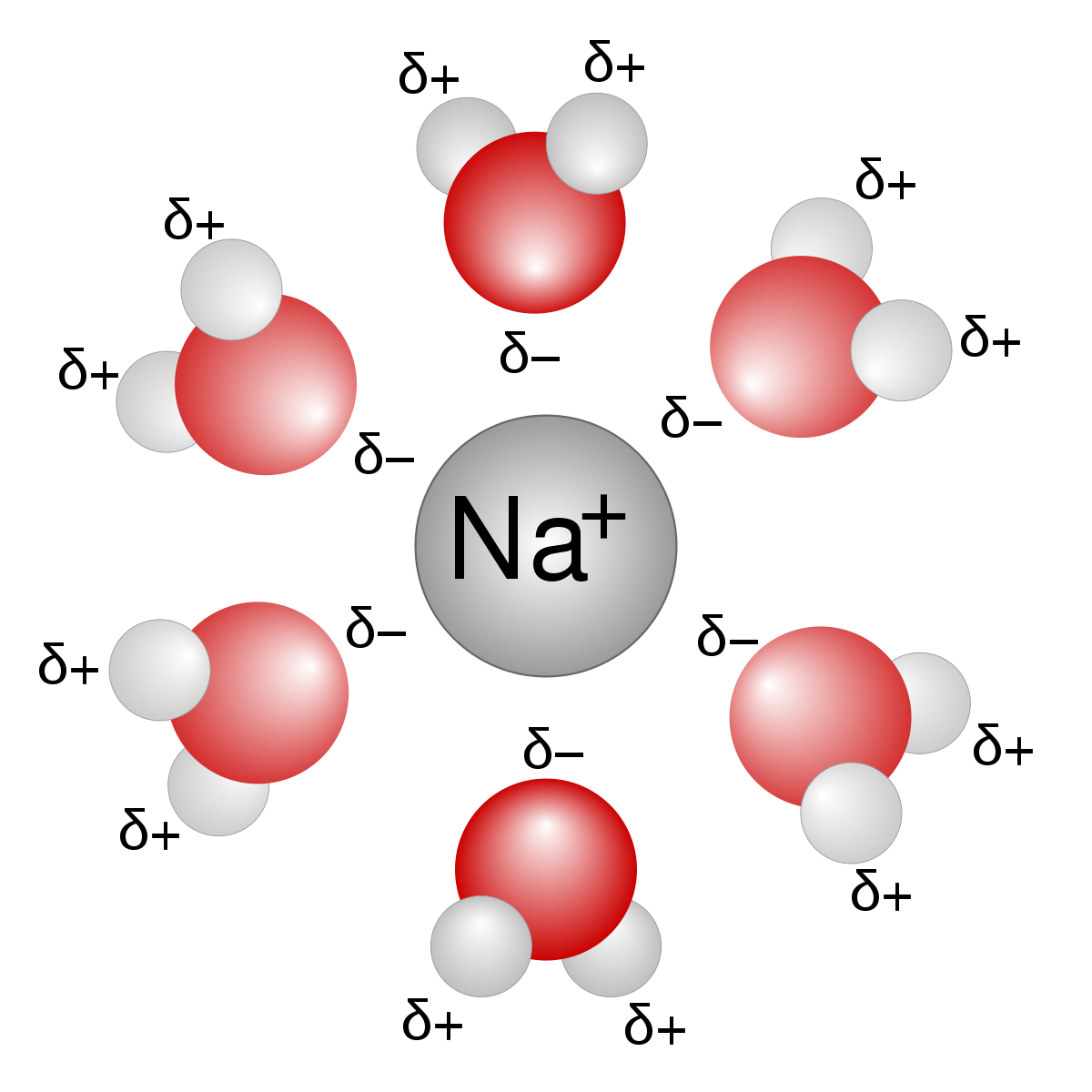
54
New cards
hydrophilic
anything that has a partial/full charge that water is attracted to (ionic and polar)
55
New cards
hydrophobic
anything without a charge that water is not attracted to
56
New cards
Mole
1 mole of any substance = Molecular weight of a molecule of that substance in grams
57
New cards
What is Avogadro’s number? Significance?
6.02 x 10^23; 1 mole of any substance has the same number of molecules as any other substance, the number being Avogadro’s number
58
New cards
Molarity
Molarity = # of moles of solute / liters of solution (ex. 1 mole of sucrose / 1 L = 1 Molar)
59
New cards
Acid
something that increases the concentration of H+ in an aqueous solution
60
New cards
Base
something that decreases the concentration of H+ in an aqueous solution
61
New cards
Which numbers on the pH scale are acidic, basic and neutral?
Acidic: Any pH between 1-7 (Higher concentration of H+ the lower pH)
Neutral: 7
Basic: Any pH between 7-14 (Lower concentration of H+ the higher the pH)
Neutral: 7
Basic: Any pH between 7-14 (Lower concentration of H+ the higher the pH)
62
New cards
Buffer
substances that resist changes in pH despite addition of acids or bases
63
New cards
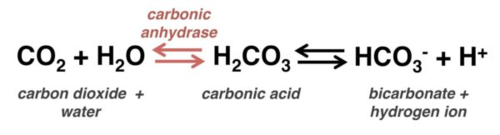
Explain the Bicarbonate Buffer System
-Carbonic Acid and Bicarbonate buffer system in our blood stream maintain blood pH of 7.4
-If pH of blood decreases, the reaction shifts to the left, to start the formation of more Carbonic Acid, which decreases the concentration of Hydrogen ions
-If pH of blood increases, the reaction shifts to the right, to start the disassociation of Carbonic Acid into Bicarbonate and Hydrogen ions, which increases the concentration of Hydrogen ions
-If pH of blood decreases, the reaction shifts to the left, to start the formation of more Carbonic Acid, which decreases the concentration of Hydrogen ions
-If pH of blood increases, the reaction shifts to the right, to start the disassociation of Carbonic Acid into Bicarbonate and Hydrogen ions, which increases the concentration of Hydrogen ions