Ionic Bonding
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
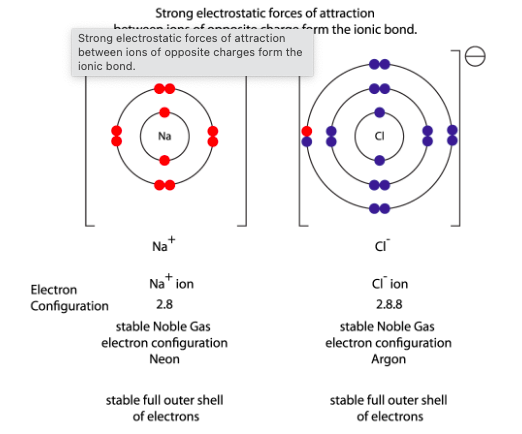
How do sodium (Na) and chlorine (Cl) form an ionic bond?
Sodium (Na) has one valence electron, while chlorine (Cl) has seven valence electrons. To achieve a stable electron configuration:
Sodium loses its one valence electron, becoming a positively charged ion (Na⁺).
Chlorine gains that electron, completing its valence shell and becoming a negatively charged ion (Cl⁻).
Because opposite charges attract, the Na⁺ and Cl⁻ ions are held together by electrostatic forces, forming an ionic bond. This process creates sodium chloride (NaCl), commonly known as table salt.
How do calcium (Ca) and chlorine (Cl) form an ionic bond, and why are two chlorine atoms needed?
Calcium (Ca) has two valence electrons, which it loses to form a Ca²⁺ ion.
Each chlorine (Cl) atom can only gain one electron, forming a Cl⁻ ion. Since calcium loses two electrons, two chlorine atoms are needed to accept both electrons.
This results in the formation of CaCl₂ (calcium chloride):
1 Ca²⁺ ion
2 Cl⁻ ions
The total charge is 2+ from calcium and 2(1−) from chlorine, which cancels out to 0, making the compound electrically neutral.
How does a chemical formula represent the formation of an ionic bond?
A chemical formula shows the elements in a compound and the ratio of their atoms.
For example, the formula CaCl₂ represents calcium chloride:
Ca (calcium) forms a Ca²⁺ ion by losing two electrons.
Cl (chlorine) forms a Cl⁻ ion by gaining one electron.
Since calcium loses two electrons, two chlorine atoms are needed to balance the charge.
The subscript "2" in CaCl₂ indicates that there are two chlorine atoms for every one calcium atom in the compound. If there is no subscript, it means only one atom of that element is present.
How are ionic compounds named?
Ionic compounds are named based on the elements involved:
The metal (cation) is named first – using its regular element name.
The nonmetal (anion) is named second – but its ending is changed to “-ide.”
For example:
NaCl → Sodium chloride
CaO → Calcium oxide
For transition metals, which can have multiple charges, a Roman numeral is used to indicate the charge:
FeCl₂ → Iron(II) chloride (because Fe²⁺ pairs with two Cl⁻ ions)
FeCl₃ → Iron(III) chloride (because Fe³⁺ pairs with three Cl⁻ ions)
For polyatomic ions (ions with multiple atoms), their special names are used:
NaNO₃ → Sodium nitrate
CaSO₄ → Calcium sulfate
How do you know if an ionic compound contains multiple atoms?
Look for more than two types of elements in the formula.
Example: NaNO₃ (Sodium nitrate) contains Na, N, and O, meaning it has a polyatomic ion (NO₃⁻).
Check for parentheses in the formula.
Parentheses indicate multiple copies of a polyatomic ion.
Example: Ca(OH)₂ (Calcium hydroxide) contains two OH⁻ ions because of the (OH)₂ notation.
Common Polyatomic Ions to Recognize:
Nitrate (NO₃⁻)
Sulfate (SO₄²⁻)
Hydroxide (OH⁻)
Carbonate (CO₃²⁻)
Phosphate (PO₄³⁻)