Monitor the rate of production of oxygen from hydrogen peroxide using manganese dioxide as a catalyst
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
Step 1
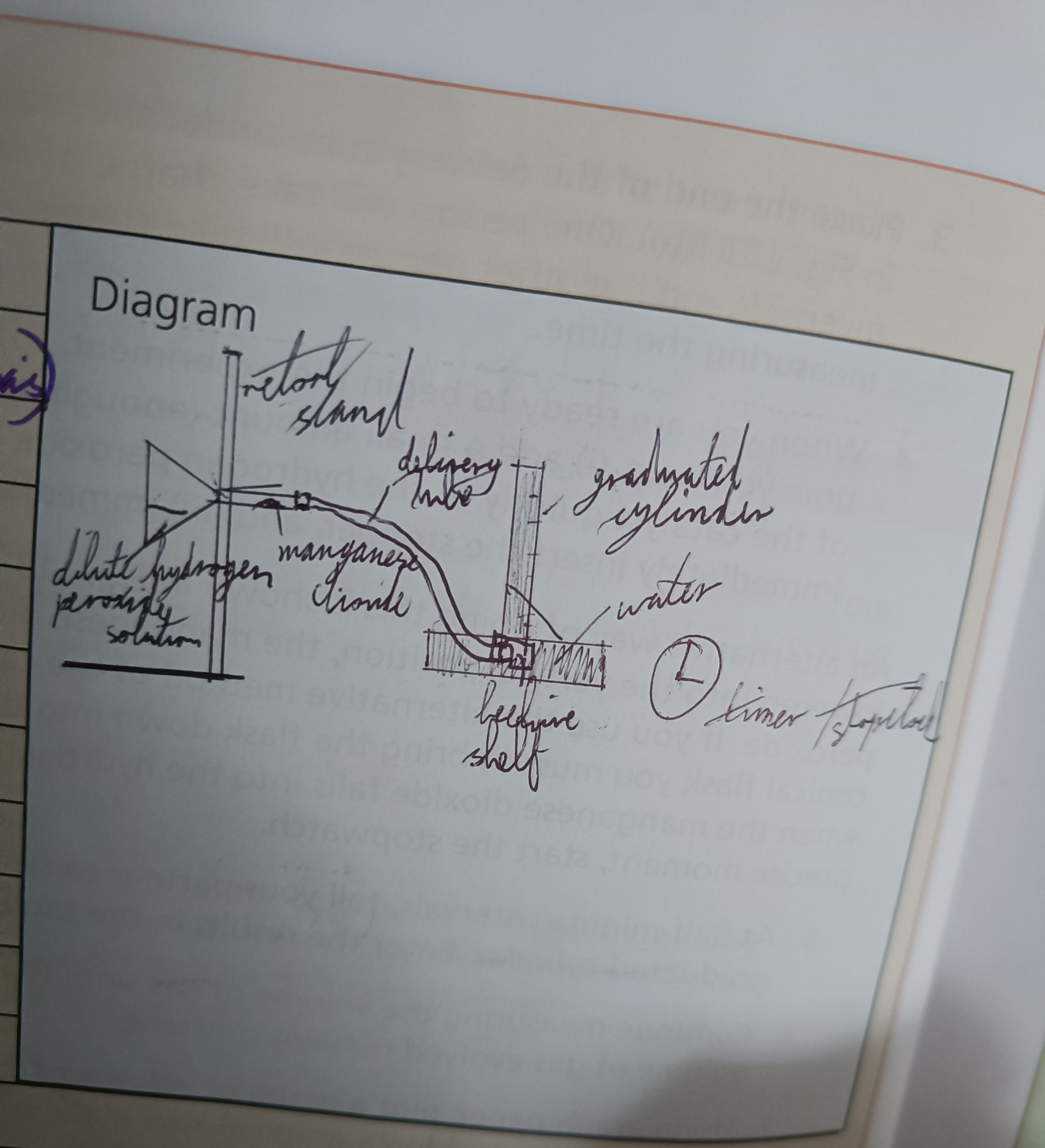
Set up apparatus as shown and bring the conical flask into the vertical position
Step 2
Start timer when manganese dioxide falls into hydrogen peroxide. Read volume of gas at half-minute intervals and repeat until reaction is done
Step 3
Plot graph of volume of oxygen vs. time
What is on the y-axis?
Volume of oxygen
What is on the x-axis?
Time
Appearance of manganese dioxide before use
Black powder
What are the products of the decomposition of hydrogen peroxide?
Water Oxygen
If hydrogen peroxide is ‘20 volume‘ what does it mean?
Certain volume of hydrogen peroxide would give off 20 times that volume when it decomposes
What property of oxygen allows collection over water?
Oxygen is only slightly soluble in water
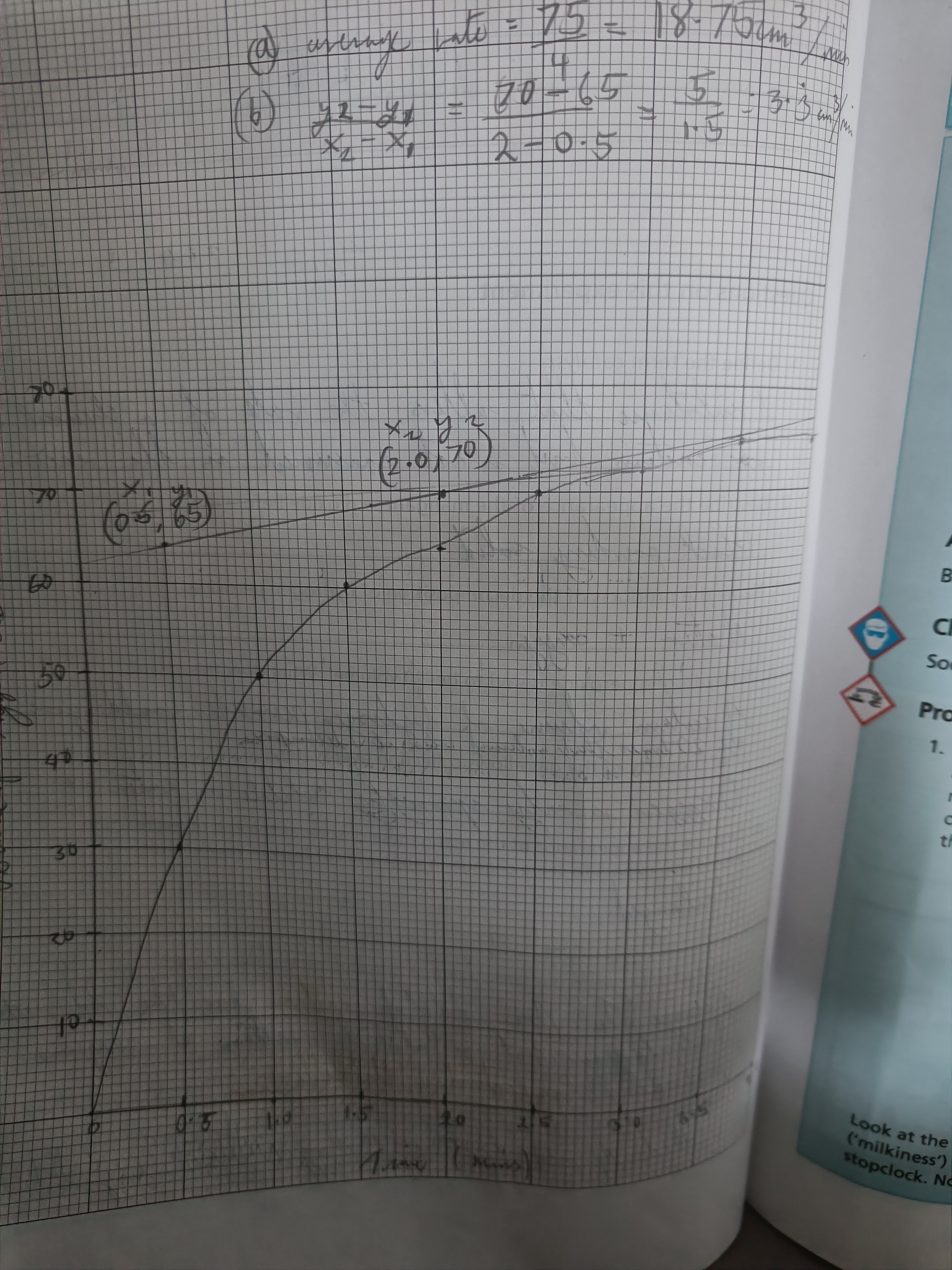
Average rate (meaning)
Mean rate of reaction for the whole reaction
One everyday use of manganese dioxide
Manufacture dry batteries e.g. AA batteries
Draw a diagram of this experiement
…
Draw a graph of this experiment finding the average rate and instantaneous rate
…