transition metal catalysis
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
why are transition metals good catalysts?
have variable oxidation states
so are able to form a range of compounds by gaining/losing e- w/in their d orbitals
in which process does vanadium act as a catalyst? give the eqns for this process:
Contact Process - industrial process for making H2SO4 :
S + O2 → SO2
SO2 + ½ O2 → (V2O5 catalyst) SO3
SO3 + H2O → H2SO4
give and explain pair of eqns for the use of V2O5 as a catalyst in the Contact Process:
V2O5 oxidises SO2 to SO3 and is itself oxidised to V2O4:
V2O5 + SO2 → V2O4 + SO3
the reduced catalyst is then oxidised back to its original state:
V2O4 + ½ O2 → V2O5
what is a heterogeneous catalyst? how do reactions occur?
catalyst which exists in a different phase from the reactants
explain how a heterogenous catalyst works:
reactants adsorb onto active sites on the surface of the heterogenous catalyst
bonds weaken/reaction takes place
products desorb from surface
give 2 egs of heterogenous catalysts and their eqns:
Fe in the Haber process:
N2 (g) + 3H2 (g) → (Fe (s) catalyst) 2NH3 (g)
V2O5 in the Contact process:
SO2 (g) + ½ O2 (g) → (V2O5 (s) catalyst) SO3 (g)
how can we minimise the cost of a reaction using a heterogenous catalyst?
maximise SA so increase no. of molecules that can react at the same time
this can be achieved by using powder/small pellets/support medium
give and explain an example of a support medium and provide the eqn:
catalytic converters contain a ceramic lattice coating in a thin layer of Rh/Pt/Pd
2CO (g) + 2NO (g) → (Rh (s) catalyst) 2CO2 (g) + N2 (g)
what is catalyst poisoning? how does this occur?
catalysts can be poisoned when impurities adsorb onto the active sites of the catalyst, blocking them
this decreases the efficiency of catalysis, increasing costs
give 2 examples of catalyst poisoning:
lead can coat the inner surface of a catalytic converter
sulfur can poison the active sites on the iron catalyst in the Haber process, forming iron sulfide (sulfur found in fossil fuels used to produce H2)
what is a homogeneous catalyst?
catalyst which exists in the same phase as the reactants (typically liquids/solutions)
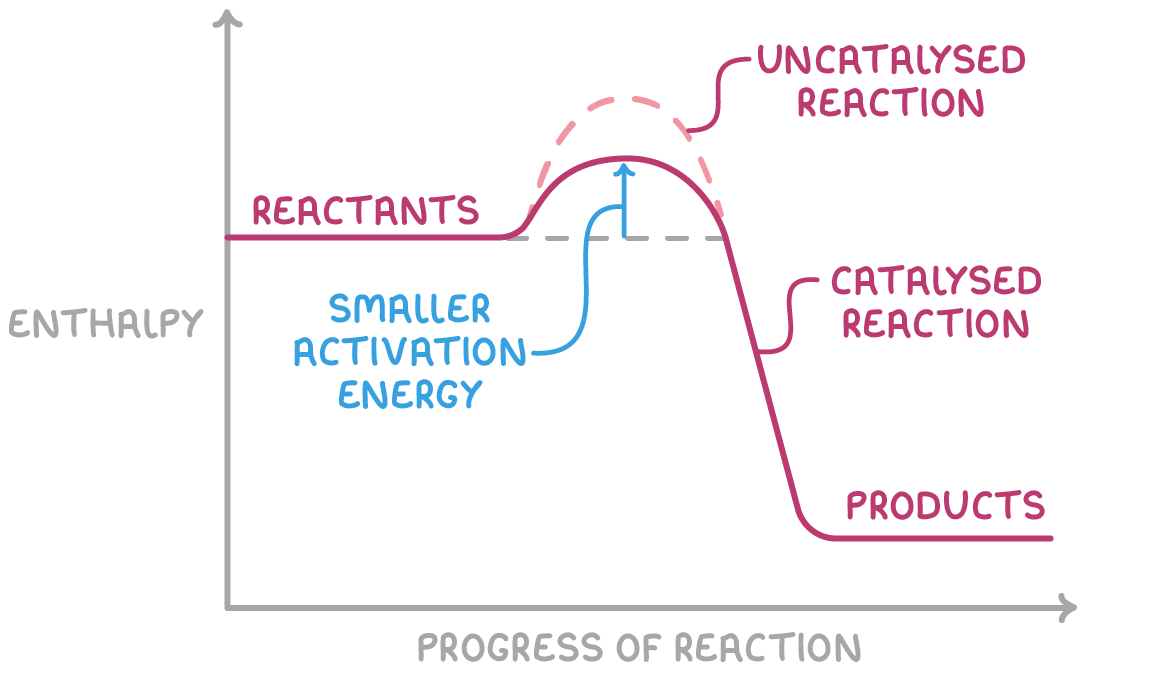
how do homogeneous catalysts work?
work by forming an intermediate species which then reacts to form the products
the EA needed to form the intermediate species is lower than that needed to make the products directly
give an example of a homogeneous catalyst and give the eqn for the reaction it catalyses:
Fe2+ ions catalyse the reaction between peronodisulfate ions and iodide ions:
S2O82- (aq) + 2I-(aq) → (Fe2+ (aq) catalyst) I2 (aq) + 2SO42- (aq)
why is the reaction between peronodisulfate ions and iodide ions slow without a catalyst?
-ve charges repel so high Ea
give a pair of eqns to show how Fe2+ acts as a catalyst in the reaction between peronodisulfate ions and iodide ions:
S2O82- (aq) + 2Fe2+ (aq) → 2Fe3+ (aq) + 2SO42- (aq)
2Fe3+ (aq) + 2I- (aq) → I2 (aq) + 2Fe2+ (aq)
other than having variable oxidation states, explain why Fe2+ ions are good catalysts for the reaction between peronodisulfate ions and iodide ions:
+ve ions attract -ve ions in catalysed process
give an example of a homogeneous catalyst which undergoes autocatalysis and give the overall eqn for the reaction it catalyses:
Mn2+ ions autocatalyse the reaction between C2O42- and MnO4- :
Mn2+ is a product of the reaction and acts as a catalyst
∴ as the reaction progresses, amount of product increases
2MnO4- (aq) + 16H+ (aq) + 5C2O42- (aq) → 2Mn2+ (aq) + 8H2O (l) + 10CO2 (g)
give a pair of ionic eqns to show how Mn2+ ions act as a catalyst in the reaction between C2O42- and MnO4- :
4Mn2+ (aq) + MnO4- (aq) + 8H+ (aq) → 5Mn3+ (aq) + 4H2O (l) (Mn2+ oxidised back to Mn3+ by MnO4-)
2Mn3+ (aq) + C2O42- (aq) → 2Mn2+ (aq) + 2CO2 (g) (Mn3+ reduced to Mn2+ by C2O42-)
how does Mn2+ lower the Ea of the reaction between C2O42- and MnO4- ?
Ea lowered because oppositely charged ions attract
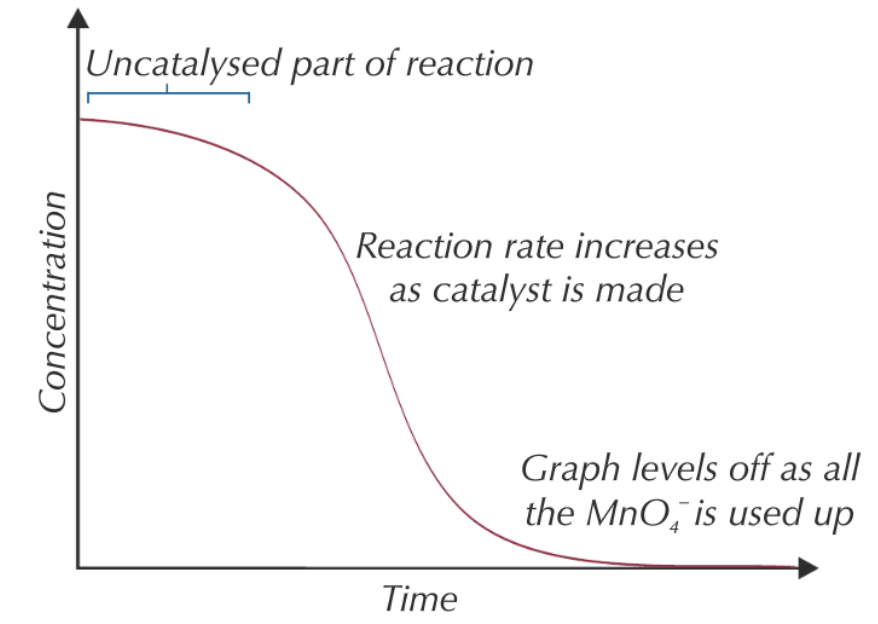
give and explain the shape of a conc-time graph of MnO4-:
initially gradient shallow as RoR low as reaction is uncatalysed as too few Mn2+ ions made ∴ high Ea
RoR increases w/ time as more of autocatalyst is made
graph begins to level off as MnO4- used up