Molecule shapes and degrees
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
2 electron pairs around a central atom
Linear molecule

3 electron pairs around a central atom (all bonded)
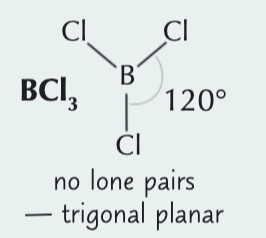
Trigonal planar
3 electron pairs around a central atom (one lone pair)
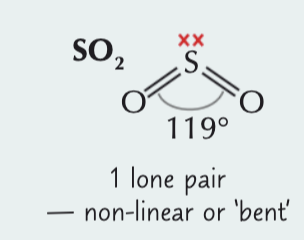
Bent
4 electrons pairs around a central atom
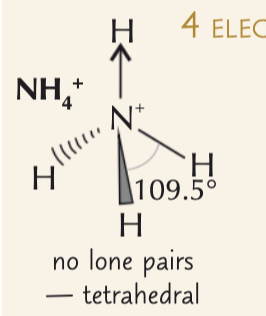
tetrahedral
4 electrons pairs around a central atom (1 lone pair)
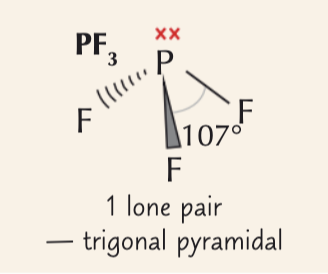
trigonal pyramidal
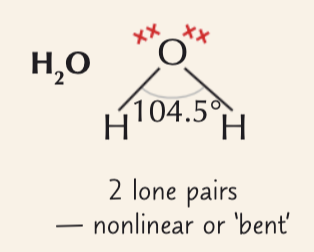
4 electrons pairs around a central atom (2 lone pairs)
bent tetrahedral
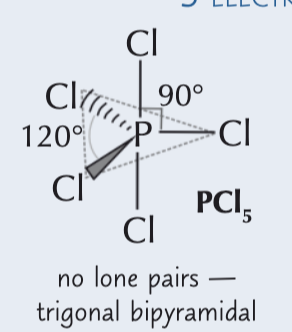
5 electron pairs around a central atom
trigonal bypyramidal
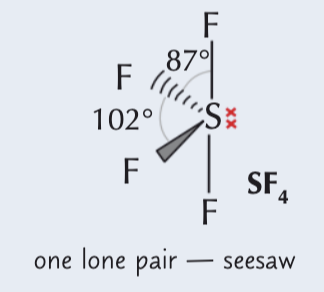
5 electron pairs around a central atom (1 lone pair)
Seesaw
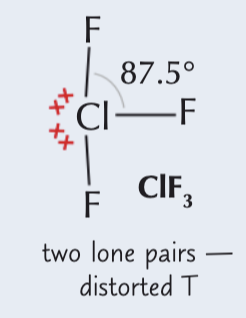
5 electron pairs around a central atom (2 lone pairs)
Distorted T
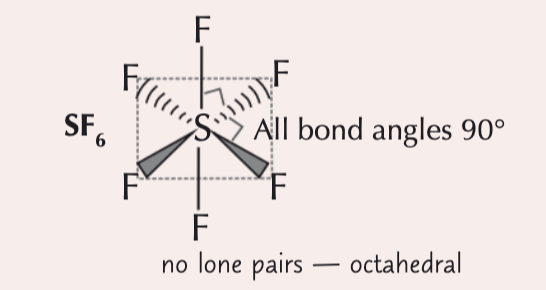
6 electron pairs around a central atom
octahedral
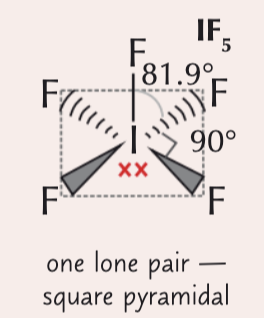
6 electron pairs around a central atom (1 lone pair)
square pyramidal
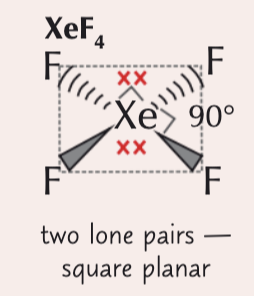
6 electron pairs around a central atom (2 lone pairs)
square planar
what does a solid line represent in a molecule diagram
A solid line represents a bond that is in the same plane as the central atom (the nitrogen atom)
what does a solid wedge represent in a molecule diagram
A solid wedge represents a bond that is coming out of the plane, towards the viewer.
what does a dashed wedge represent in a molecule diagram
A dashed wedge (or a series of dashes) represents a bond that is going into the plane, away from the viewer.