3. Protein Structure and Function
1/62
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
63 Terms
What are common functions of proteins?
Structure, movement, catalysis, transport, and communication
A protein’s function is determined by its ______
structure
What determines the 3D shape of a protein?
Its sequence (how the amino acids are arranged)
What forces stabilize the 3D shape of a protein and mediates its interactions?
Non-covalent bonds (ie. H-bonds, Van der Waals, etc)
At a pH above the pKa, the acid exists predominately as ________
A- (deprotonated/conjugate base)
At a pH below the pKa, the acid exists predominately as ________
AH (protonated/acid)
When the pH = pKa the acid exists as ______
AH/A- (an equilibrium of protonated/deprotonated and conjugate base/acid)
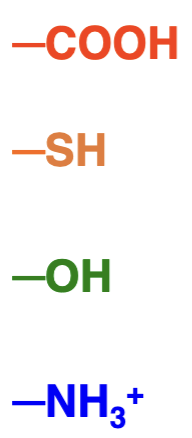
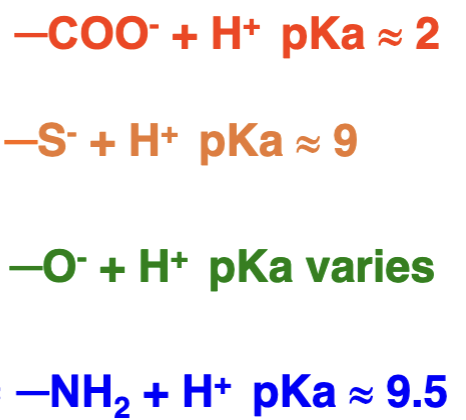
What products are these molecules in equilibrium with and what are their pKa values?
True or false: the chemical environment does not affect the pKa of acidic groups.
False
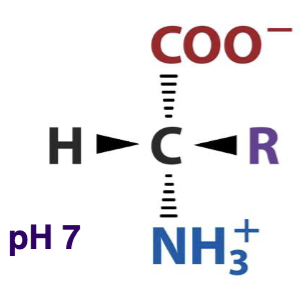
The structure of a general amino acid at pH 7 is shown below. What does this AA look like at pH 1?
protonated/acidic form
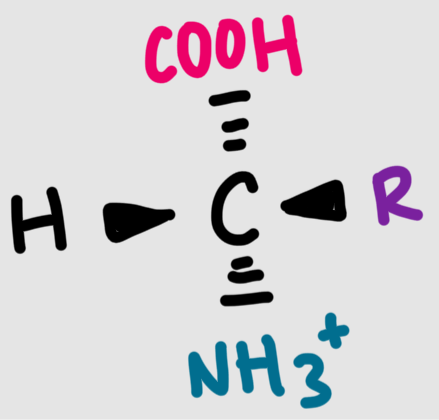
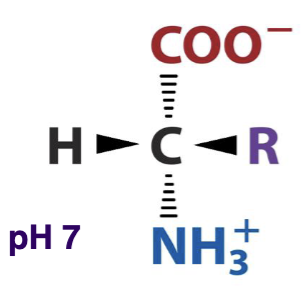
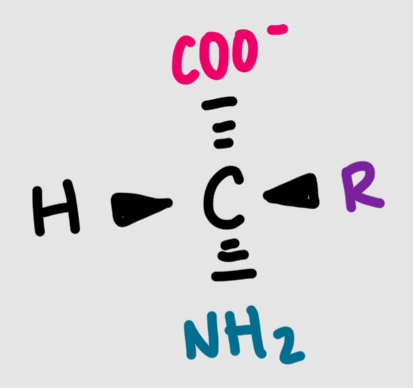
The structure of a general amino acid at pH 7 is shown below. What does this AA look like at pH 14?
deprotonated/conjugate base form
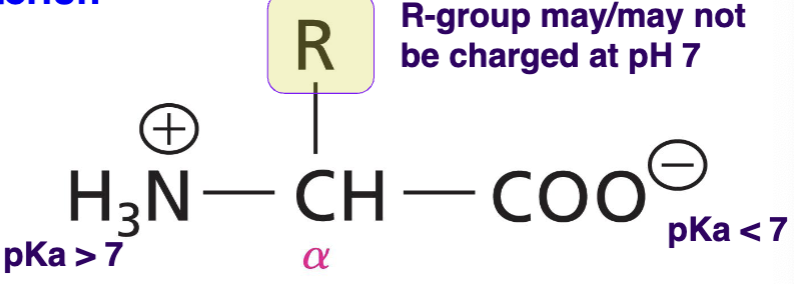
Zwitterion
molecule containing an equal number of positively and negatively charged functional groups
What is the structure of a general amino acid at pH 7?
alpha carbon with an amino group, a carboxyl group, and a side chain (R)
Chiral molecules
Cannot be superimposed on its mirror image. Asymmetric property.
What conditions are required for a C to be a chiral molecule?
Must have four groups attached to it
All four groups must be different
Amino acid classifications are based on ______
Side chains only (R group)
Hydrophobic classification
non-polar
Polar classification
Uncharged (0) at pH 7
Charged classification
Most polar
Hydrophobic amino acids lack _____ functional groups and have mainly ____ side chains
reactive, hydrocarbon
What are the 8 hydrophobic amino acids?
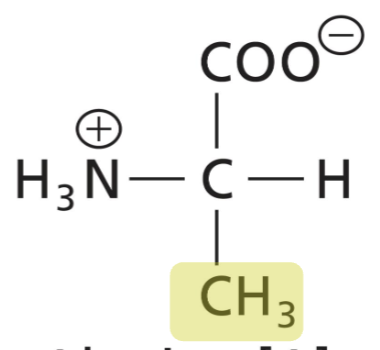
Alanine (Ala)
Valine (Val)
Leucine (Leu)
Isoleucine (Ile)
Phenylalanine (Phe)
Tryptophan (Trp)
Methionine (Met)
Proline (Pro)
Alanine (Ala)
Aliphatic R, hydrophobic (interactions)
Valine (Val)
Aliphatic R, highly hydrophobic (interactions)
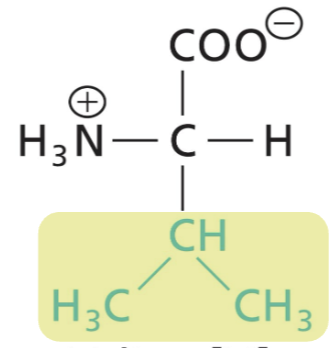
Leucine (Leu)
Aliphatic R, highly hydrophobic (interactions)
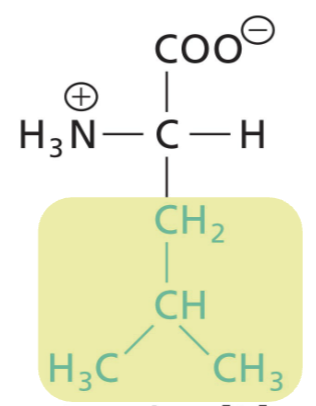
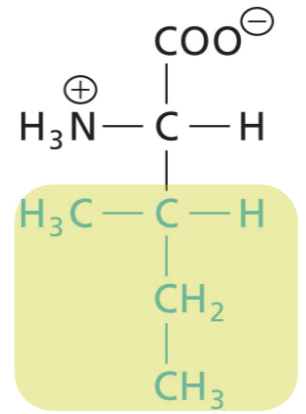
Isoleucine (Ile)
Aliphatic R, highly hydrophobic (interactions)
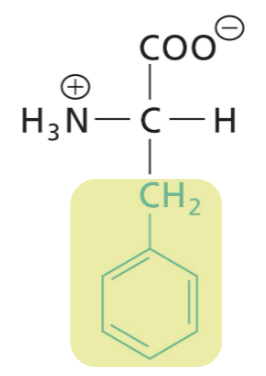
Phenylalanine (Phe)
Aromatic R (weakly absorbs UV light), highly hydrophobic (interactions)
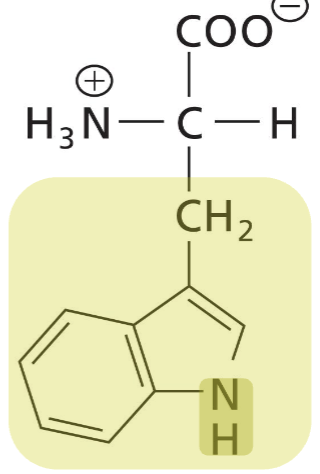
Tryptophan (Trp)
Aromatic R (strongly absorbs UV light), heterocyclic, hydrophobic and bulky R, hydrophobic interactions (acts as H-donor in H-bonds)
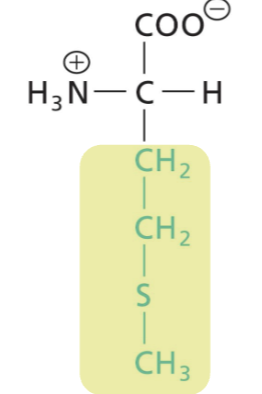
Methionine (Met)
Hydrophobic (interactions), sulfure side group (thioether)
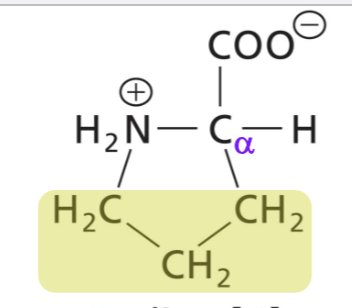
Prolin (Pro)
Aliphatic side chain, secondary amino group, hydrophic (interactions)
What are the 8 polar amino acids?
Glycine (Gly)
Serine (Ser)
Threonine (Thr)
Asparagine (Asn)
Glutamine (Gln)
Tyrosine (Tyr)
Cysteine (Cys)
Histidine (His)
Which polar amino acids have ionizable R groups? How is this determined.
Tyr, Cys, and His because their pKa values are in the range 1-14.
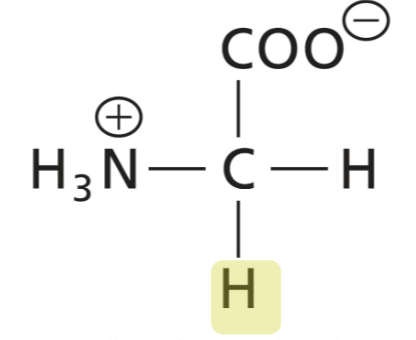
Glycine (Gly)
Achiral, weakly polar, and very small
Serine (Ser)
Polar, uncharged, hydroxyl group, will act as H-bond donor and acceptor
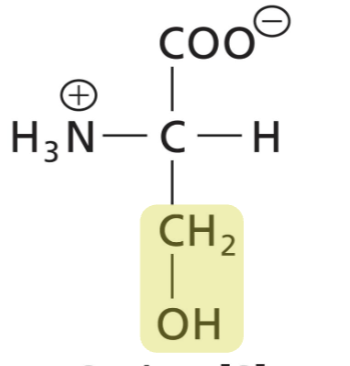
Threonine (Thr)
Polar, uncharged, hydroxyl group, H-bonds can be donor or acceptor. Can be phosphorylated
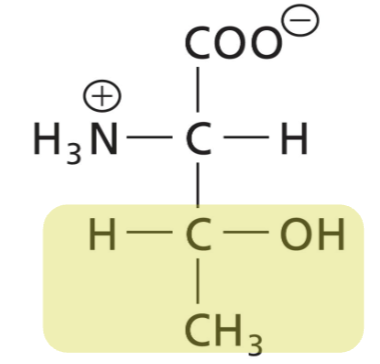
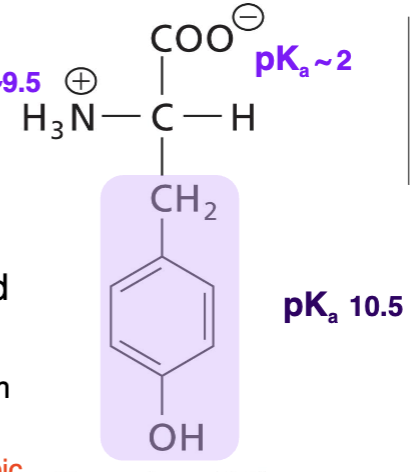
Tyrosine (Tyr)
weakly polar, uncharged, aromatic R absorbs UV lights @280. Can participate in hydrophobic interactions. Hydroxyl group. H bonds can accept and donate. Can be phosphorylated.
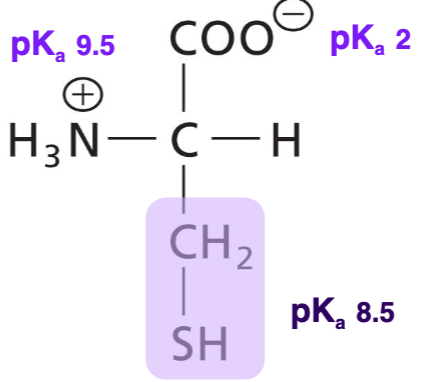
Cysteine (Cys)
polar, uncharged, sulfur side group (sulfyhydryl), H-bonds usually donating. Forms disulphide bonds with another Cys
Describe the conditions needed for formation of a disulphide (R-S-S-R) bond in a protein.
The 2 Cys residues must be close enough to each other
Oxidizing environment (extracellular)
Cystine
Two cysteine molecules joined by a disulphide bond. Pretty hydrophobic
Asparagine (Asn)
Amide side chain, polar, non-charged, H-bonds as donor and acceptor
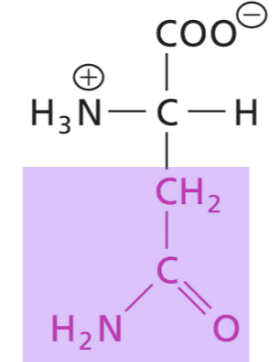
Glutamine (Gln)
Amide side chain, polar, non-charged, H-bonds as donor and acceptor
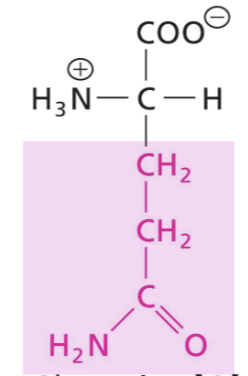
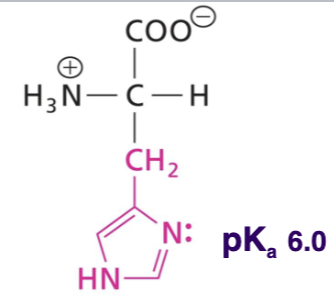
Histidine (His)
Side chain uncharged at neutral pH, aromatic weakly absorbs at 280nm, polar, H-bond donor or acceptor
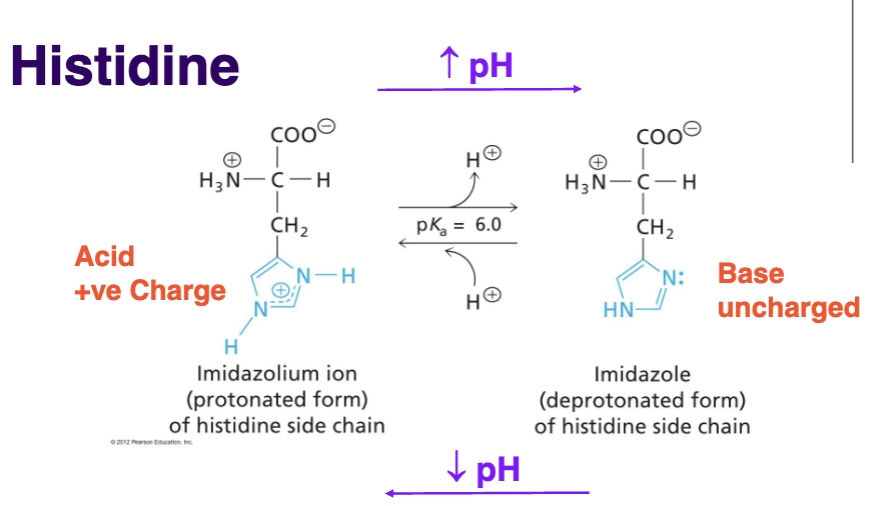
Describe other unique properties of Histidine
Can act as acid or base, ionization state depends on chemical environment, can accept or donate a proton (forms H-bonds),
What are the 4 charged amino acids?
Aspartate (Asp)
Glutamate (Glu)
Lysine (Lys)
Arginine (Arg)
What other properties do ALL of the charged amino acids have?
They are ALL polar molecules but are NOT classified in the polar AA
They ALL have charged R groupa at pH 7
Aspartate (Asp)
Negatively charged R at pH 7, second carboxyl group on extra C, acidic AA, very polar, H-bond acceptor
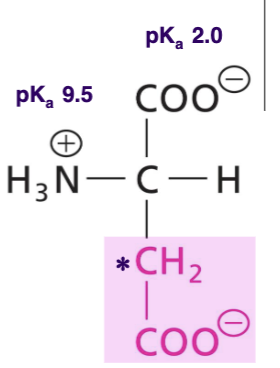
Glutamate (Glu)
Negtaive charged R at pH 7, second carboxyl group on two extra C, acidic AA, very polar, H-bond acceptor
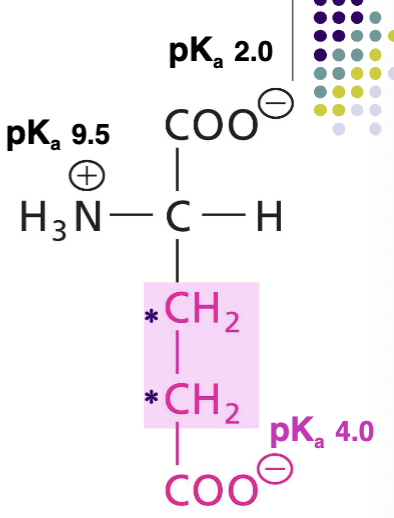
Lysine (Lys)
Positively charged R at pH 7, basic AA, additional amono group (R), , H-bond donor, very polar
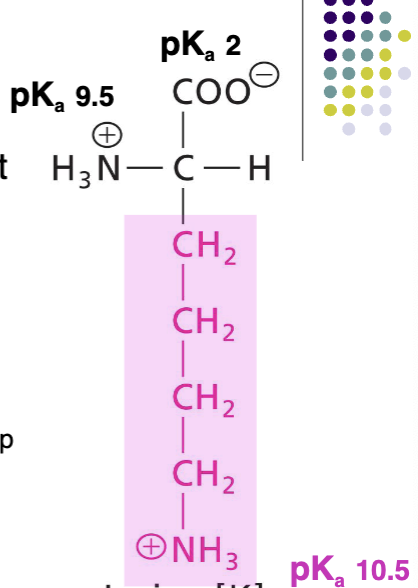
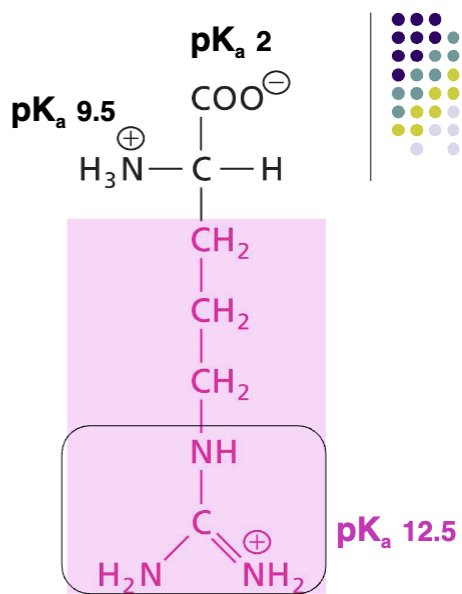
Arginine (Arg)
Positively charged R at pH 7, basic AA, never deprotonated, very polar, H-bond donor
Where are uncharged polar side chains usually located?
Protein surface. To occur in interior, must group to H-bond with.
Where are charged polar side chains usually located?
Protein surface. To occur in interior, must have a group with an opposite charge
True or false: All amide bonds are peptide bonds.
False, all peptide bonds are amide bonds but not all amide bonds are peptide bonds. Peptide bonds must join two AA together.
What charge does a peptide bond have?
0 at pH 7
Formation of a peptide bond
Through dehydration reaction: COO- of one AA is joined to the NH3+ of another AA. The starting AA will have the N-terminus and the end AA will have the C-terminus
True or false: The carbonyl and amino group in a peptide bond will not ionize as pH changes between 1-14.
True, they remain neutral to maintain an overall 0 charge on the bond as a whole
A dipeptide results in __ AA residues.
2
True or false: Ser-Ala and Ala-Ser are the same thing.
False: In Ser-Ala, Ser has the N terminus and Ala has the C terminus. It becomes the opposite in Ala-Ser.
True or false: Peptide bonds and proteins run from N-terminus to C-terminus.
Flase: Peptide bonds run C → N and proteins rune N → C
How many peptide bonds are in a tetrapeptide?
3 (always one less that the number of AA joined togther)
What is different about the terminal a-amino and a-carboxyl groups in a peptide?
They retain their charges (+ and -) while the rest of the groups lose their charges when peptide bonds are formed. R group will maintain their charge.
What stabilizes the primary structure of polypeptides?
Covalent peptide bonds
Characteristics of peptide bonds
Covalent
Amide
No Charge
Polar
Abs UV light at 230 nm
Formed by dehydration/condensation reaction
e- are delocalized: have resonance with aprtial double bond character and no rotation around C-N bond (rigid and planar)
Functional groups can be H-bond donors or acceptors
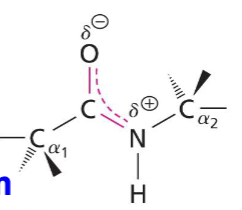
Backbone of polypeptides
a-Carbons atoms and those involved in the peptide bond (C, N, O, H)
CONTINUE SECONDARY STRUCTURE