Biology: Biological molecules
1/60
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
61 Terms
2.1 Introduction to biological molecules
2.1 Introduction to biological molecules
Types of biological molecules
The cells of all living organisms primarily consist of four types of molecules: carbohydrates, lipids, proteins, and nucleic acids. These biological molecules are organic, meaning they contain the element carbon.
These molecules also contain additional elements:
Carbohydrates - Carbon (C), hydrogen (H), and oxygen (O).
Lipids - Carbon (C), hydrogen (H), and oxygen (O).
Proteins - Carbon (C), hydrogen (H), oxygen (O), and nitrogen (N).
Nucleic acids - Carbon (C), hydrogen (H), oxygen (O), nitrogen (N), and phosphorus (P).
Monomer and polymer
Monomer - Smaller units that combine to make a large molecule (polymer).
Polymer - Large molecule made up of many repeating units of monomers joined together by chemical bonds.
The process by which monomers join to form a polymer is known as polymerisation.
Condensation and hydrolysis reactions
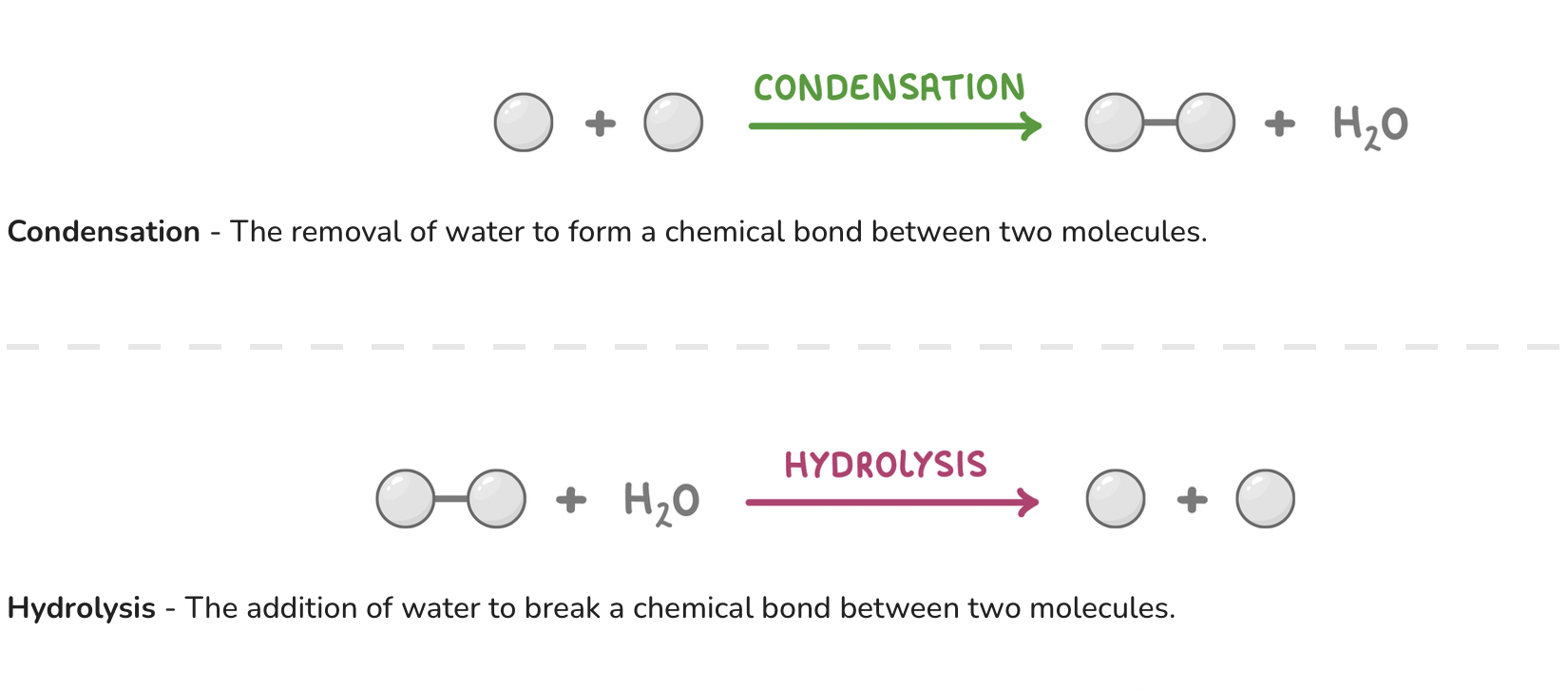
2.2 Inorganic ions
2.2 Inorganic ions
Key definitions
Ion - An atom with an electric charge.
Inorganic ion - An ion that does not contain carbon (with some exceptions).
Cation - An ion with a positive charge.
Anion - An ion with a negative charge.
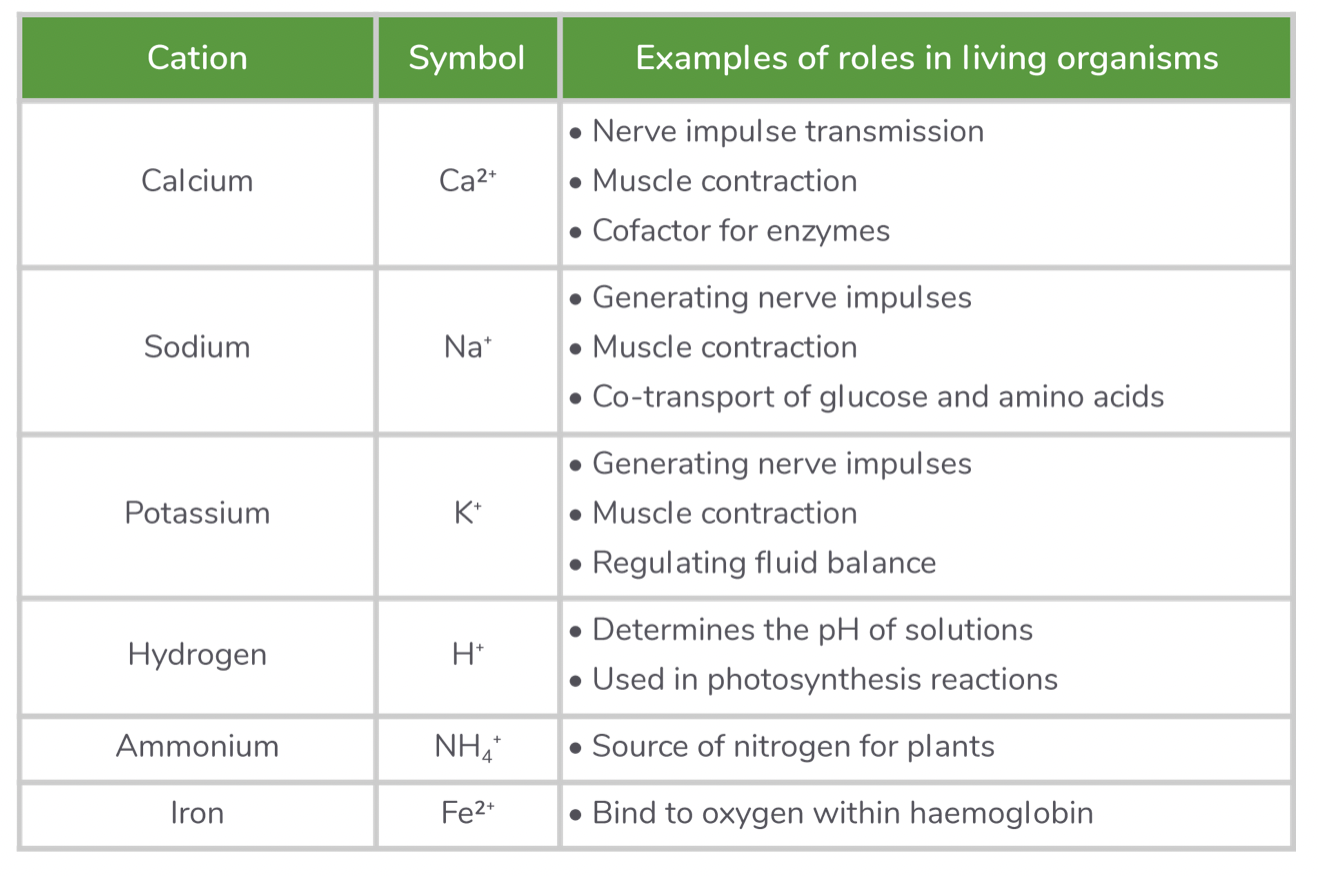
Cations
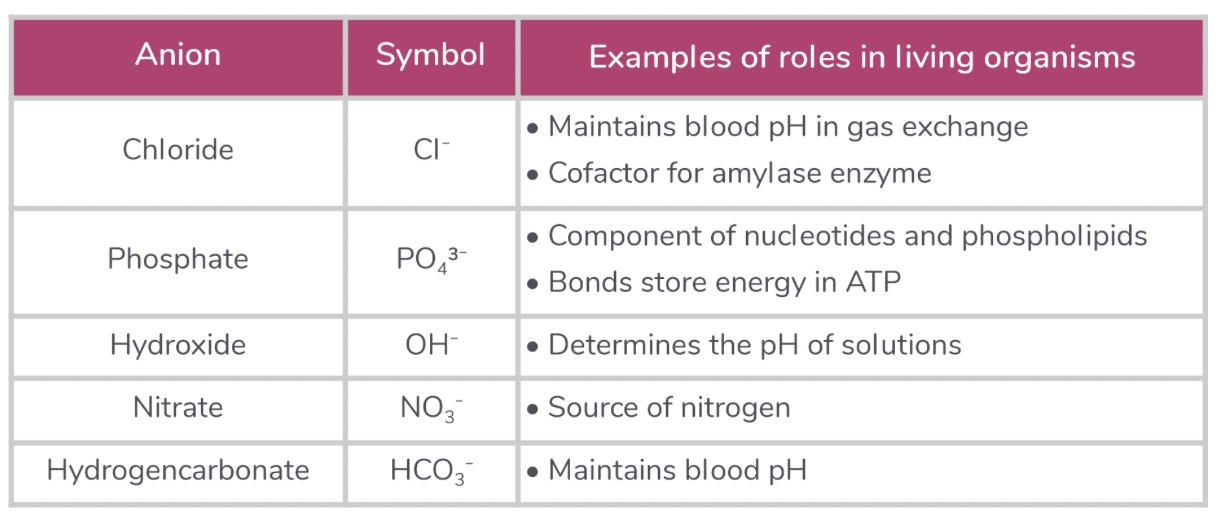
Anions
2.3 Water
2.3 Water
Structure of water
A molecule of water (H2O) is made up of one oxygen atom (O) joined to two hydrogen atoms (H). These atoms are held together by two covalent bonds.
Oxygen shares one electron with each hydrogen atom while each hydrogen atom shares its one electron with oxygen.
The shared electrons are pulled towards the oxygen atom, giving the oxygen atom a slightly negative charge (δ-). This leaves the hydrogen atoms with a slightly positive charge (δ+). This means that water has both positive and negative poles, making it a dipolar molecule.
Hydrogen bonding in water
Each water molecule consists of a partially negative oxygen end and a partially positive hydrogen end. This causes water molecules to interact with one another.The partially positive hydrogen end of one water molecule attracts towards the partially negative oxygen end of another molecule. This force of attraction is known as a hydrogen bond.These hydrogen bonds form between many water molecules, causing them to stick together and giving water some of its useful properties.
Roles of water
Solvent - Many substances dissolve in water.
Temperature control - Water can buffer sudden temperature changes.
Cooling mechanism - Mammals use the evaporation of water in sweat to cool the skin.
Habitat - Many organisms can survive and reproduce in water.
Metabolite - Many chemical reactions involve water.
Transport - Organisms can use water to move substances.
Water as a solvent
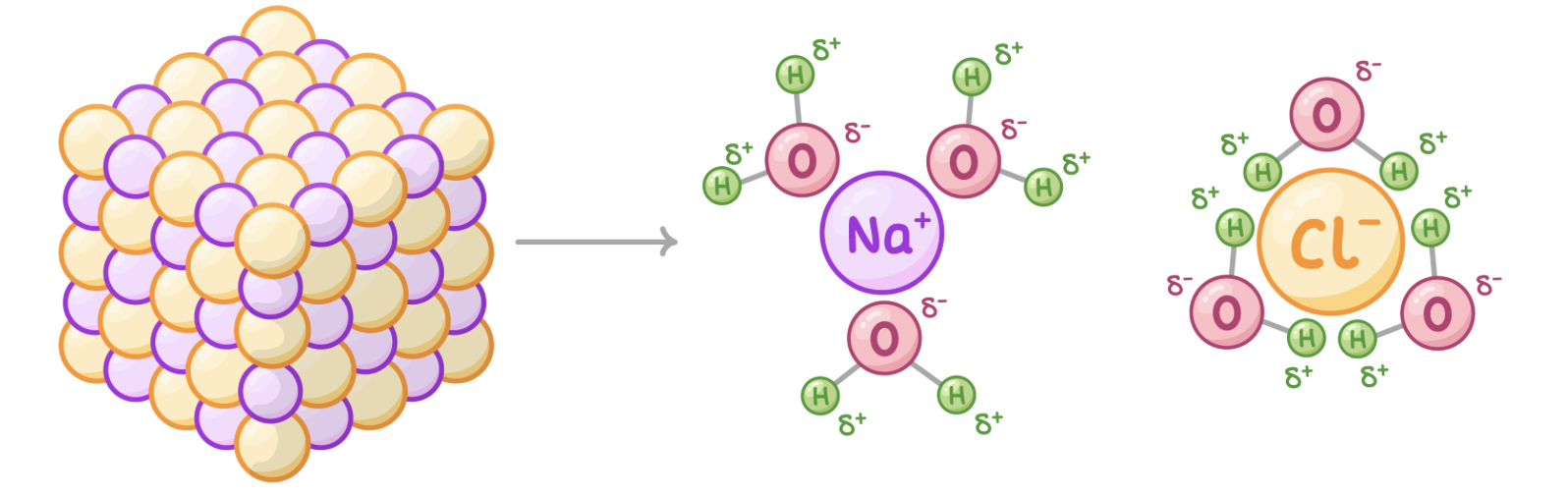
Many substances within cells are ionic compounds. This means they consist of positive and negative ions (e.g. salt is made up of Na+ and Cl- ions). When these ionic compounds are added to water, the ions are split apart.
As water is polar, the slightly negative oxygens are attracted to the positive ions whilst the slightly positive hydrogens are attracted to the negative ions.
Each ion is surrounded by water molecules and the compound dissolves.
Water as a temperature buffer
Water has a high specific heat capacity, which means a lot of energy is needed to raise the temperature of 1 gram of water by 1°C.
The many hydrogen bonds between water molecules can absorb a lot of energy before being broken, so it takes a lot of energy to break the hydrogen bonds and heat the water.
The high specific heat capacity of water means that it is resistant to rapid changes in temperature. As many organisms are made up of water, this allows the body to remain at a fairly stable temperature.
Note: Sometimes, specific heat capacity is defined in terms of 1 kilogram of water instead of 1 gram. It doesn't matter which you use as long as it's just 1 unit of water, but make sure that you use the units you've been asked for in any calculations.
Water as a cooling mechanism
Hydrogen bonding between water molecules also means that a lot of energy is needed to evaporate 1 gram of water.
This means that water has a high latent heat of vaporisation (a high boiling point). A lot of energy is required to break the hydrogen bonds to change it from a liquid to a gas.
This is useful for organisms because they can use evaporation of water as a method of cooling without losing too much water. When water evaporates from the surface of the skin, it takes heat energy away from the surface, cooling the organism down.
Water as a habitat
Since water has a high specific heat capacity and a high latent heat of vaporisation, it does not change temperature or evaporate easily. This provides a stable environment for many organisms to live in.
However, at low temperatures, water freezes to form ice. Water molecules are held further apart in ice, making it less dense than water.
This causes ice to float, forming an insulating layer at the surface of ponds and lakes. This means the water below this layer does not freeze, allowing organisms within the water to move and survive.
Water as a metabolite
Water is involved in many chemical reactions inside organisms.
Hydrolysis reactions - These use water to break down complex molecules.
Condensation reactions - These release water to join molecules together.
Photosynthesis - This uses water as a raw material.
Water as a transport medium
The tendency of water molecules to stick together (via hydrogen bonds) is known as cohesion. Water also has a tendency to stick to other materials; this is known as adhesion.
Strong cohesion and adhesion helps water to flow through organisms, carrying substances along with it.
In the same way, when water molecules meet air they create a high surface tension. This forms a skin-like structure at the surface of the water which is strong enough to support small organisms such as pond-skaters.
2.4 Carbohydrates introduction
2.4 Carbohydrates introduction
What are carbohydrates
Carbohydrates are biological molecules that contain the elements carbon (C), hydrogen (H), and oxygen (O).
'Carbo' - Contains the element carbon.
'Hydrate' - Contains hydrogen and oxygen atoms, typically in a ratio of 2:1 like water (H2O) in most simple carbohydrates.
The general formula for a carbohydrate is Cx(H2O)y.
Role of carbohydrates
Energy supply for cells - This is the main role of carbohydrates.
Energy storage - Sugars can be stored as complex carbohydrates (e.g. starch or glycogen).
Structural components - Cellulose and chitin are used in cell walls.
Cellular recognition - Glycoproteins help cells identify each other and communicate.
Building blocks for biological molecules - Deoxyribose and ribose can be used to make nucleic acids.
Types of carbohydrates
2.5 Types of carbohydrates
2.5 Types of carbohydrates
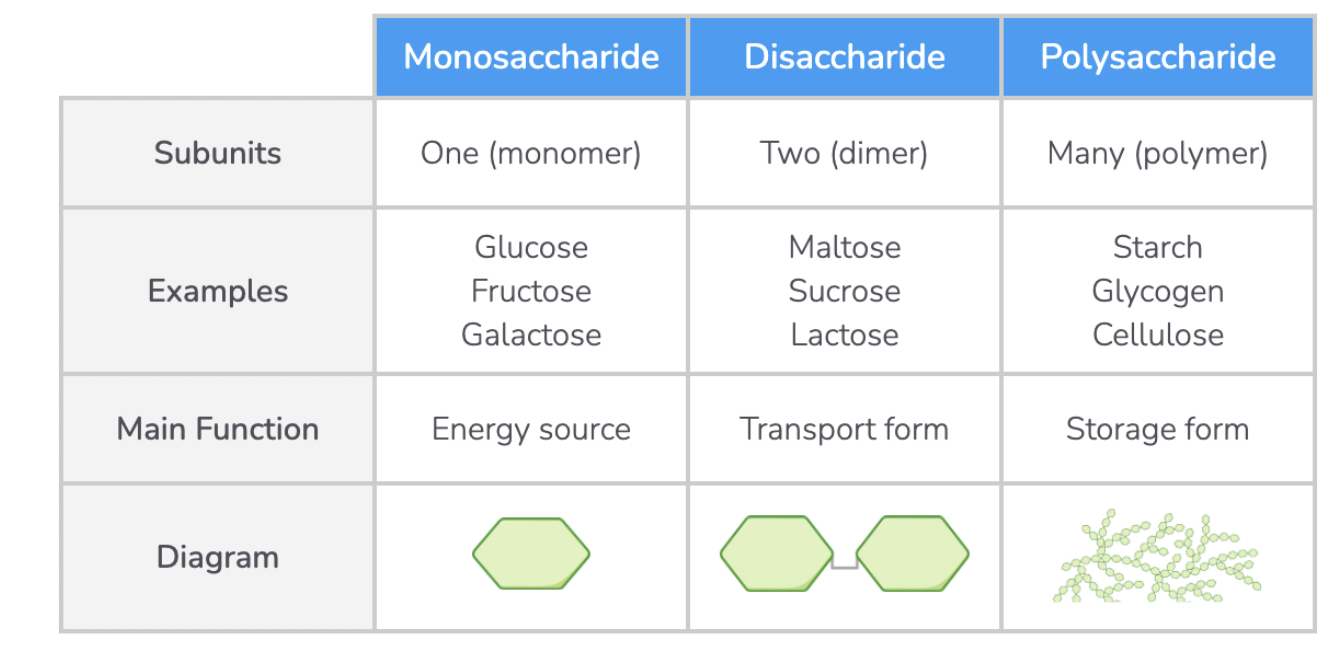
Monosaccharides
Monosaccharides are the simplest form of carbohydrates, also known as 'simple sugars'. Monosaccharides are soluble, sweet-tasting and are found in many foods such as fruits, vegetables, and grains.
They have the general formula (CH2O)n where 'n' can be any number from 3 to 7.
Pentose sugars (5 carbon atoms)
Ribose
Deoxyribose
Hexose sugars (6 carbon atoms)
Glucose
Fructose
Galactose
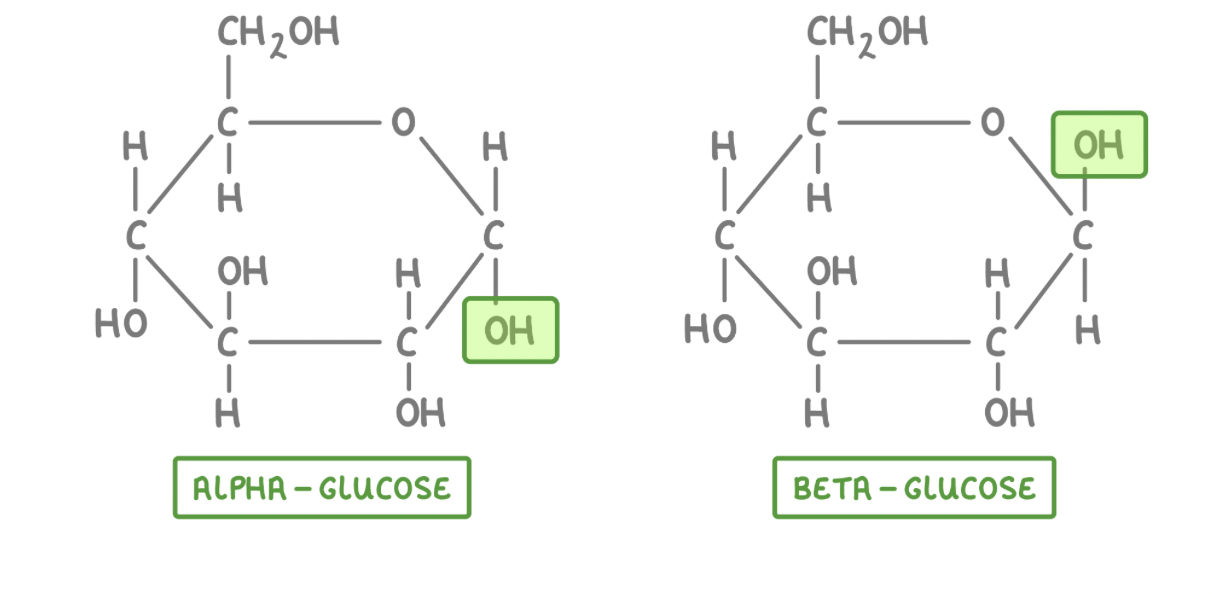
Alpha glucose and Beta glucose
Glucose is a hexose (6-carbon) sugar with the formula C6H12O6. The atoms in glucose can be arranged in two different ways.
This means that there are two isomers of glucose:
Alpha-glucose (α-glucose)
Beta-glucose (β-glucose)
The only difference between the two forms is the orientation of the hydroxyl group (OH) on carbon 1 (the first carbon atom in the ring).
Properties and uses of glucose
Glucose is used as the primary energy source in animals and plants.
The following features of glucose help it to function as an energy source:
It is soluble - The hydroxyl groups can form hydrogen bonds with water, so it can be transported around organisms.
Its bonds store lots of energy - This energy is released when the bonds are broken.
Disacharrides
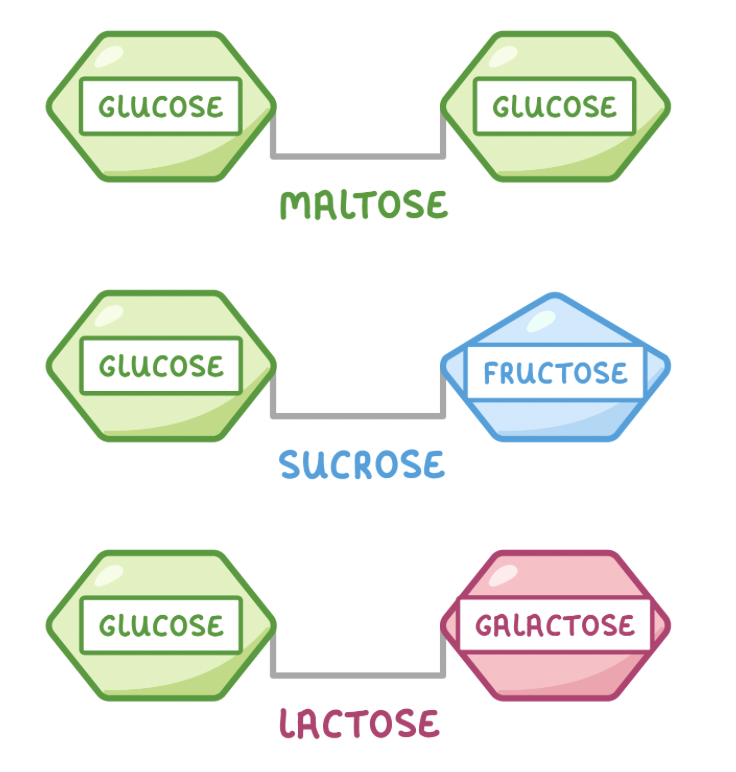
Disaccharides are formed when two monosaccharides join together. Examples of disaccharides include maltose (found in grains and cereals), sucrose (used as a transport sugar in plants), and lactose (the main carbohydrate found in milk)
Maltose is made up of glucose joined to glucose.
Sucrose is made up of glucose joined to fructose.
Lactose is made up of glucose joined to galactose.
Disaccharide formation and breakdown
Disaccharides are created via condensation reactions, and broken down via hydrolysis reactions. These reactions involve the formation or the breakdown of a covalent bond known as a glycosidic bond.
Condensation reaction
When two monosaccharides join, a hydroxyl group (OH) of one monosaccharide reacts with a hydroxyl group (OH) of another monosaccharide. This forms a glycosidic bond, and a water molecule (H2O) is released.
In the image above, the hydroxyl groups on carbons 1 and 4 are reacting together, so a 1-4 glycosidic bond forms, but they can also form between other carbons.
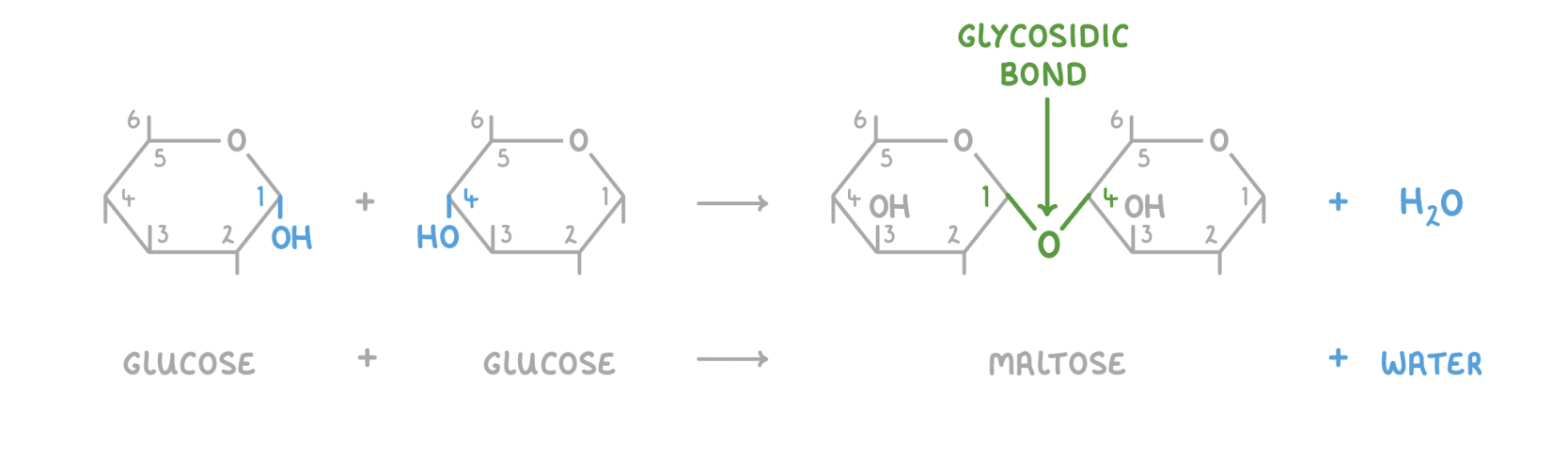
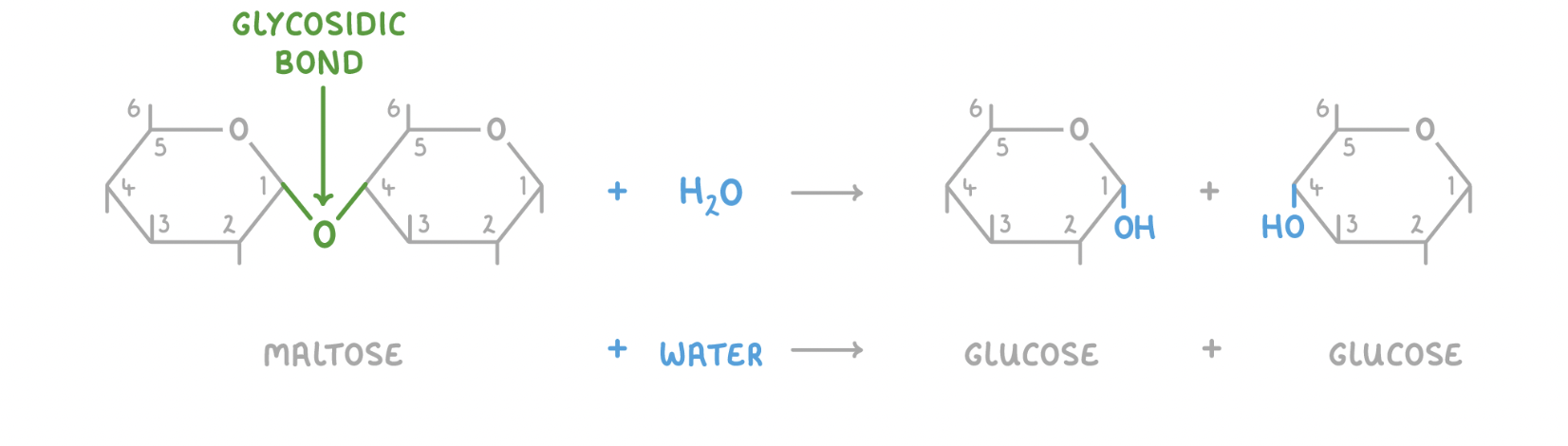
Hydrolysis reaction
When a water molecule (H2O) is added to a disaccharide, the glycosidic bond is broken to release the 2 monosaccharides.
2.6 Carboydrates
2.6 Carbohydrates
Polysaccharides
Polysaccharides are complex carbohydrates made up of many monosaccharides joined via glycosidic bonds.
Examples of polysaccharides include starch, glycogen, and cellulose.
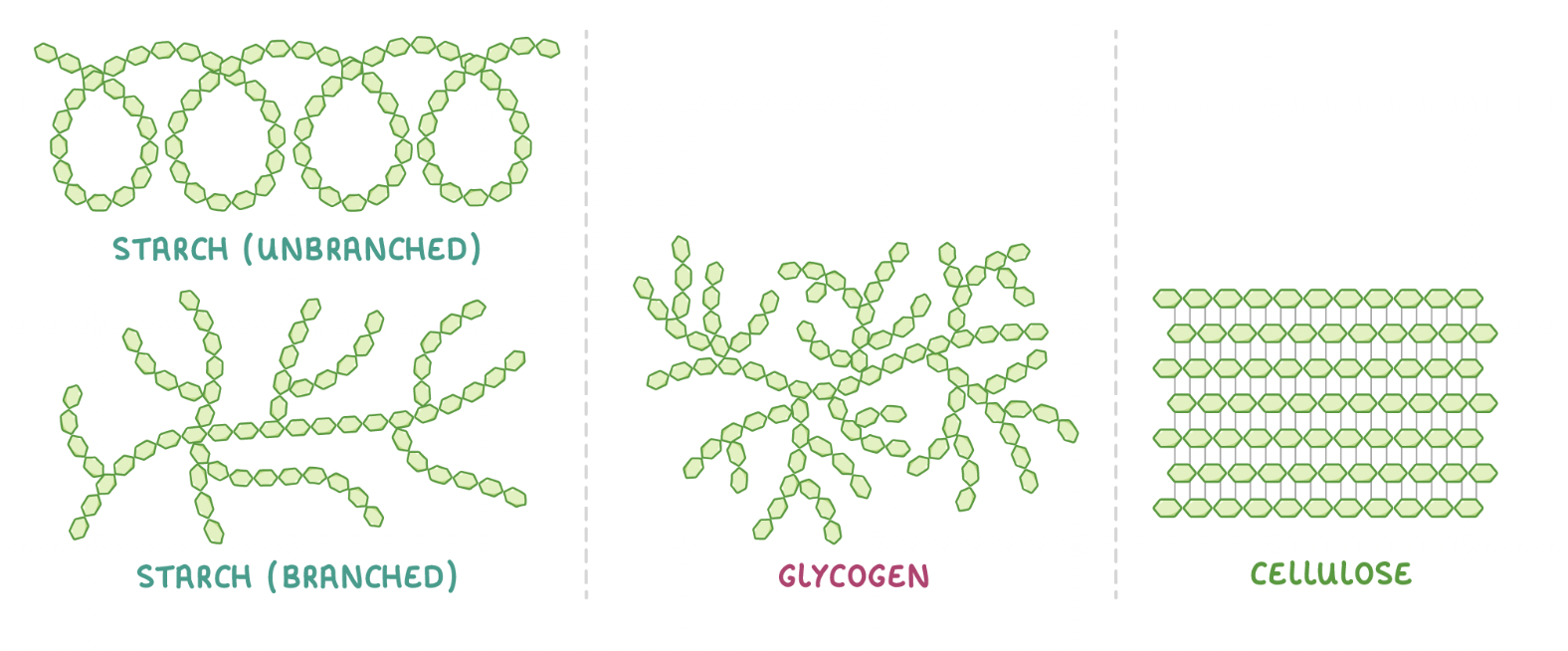
Starch
Starch is an example of a polysaccharide used by plants to store excess glucose. This means that starch can be hydrolysed back into glucose when plants require energy.
Starch is made up of many alpha-glucose monomers joined via glycosidic bonds to form chains. Starch chains come in two forms: amylose and amylopectin.
The following features of starch help it to function as a store of energy:
Insoluble - It does not affect the water potential, so water does not enter cells by osmosis.
Large - It cannot diffuse out of cells.
Many side branches - These allow enzymes to hydrolyse the glycosidic bonds easily to rapidly release glucose.
Coiled - This makes it compact so that a lot of glucose can be stored in a small space.
Hydrolysis releases alpha-glucose monomers - These are readily used in respiration.
Amylose
Amylose is a long, unbranched chain of alpha-glucose joined by 1-4 glycosidic bonds. The angles of these bonds cause the chain to coil into a helix to make a compact structure
Amylopectin
Amylopectin is a long, branched chain of alpha-glucose joined by both 1-4 and 1-6 glycosidic bonds. Its side branches allow enzymes to hydrolyse alpha-glucose monomers easily.
Glycogen
Glycogen is an example of a polysaccharide used by animals to store excess glucose. This means that glycogen can be hydrolysed back into glucose when animals require energy.
Glycogen is very similar to starch, but it is used by animals rather than plants.
Glycogen is made up of many alpha-glucose monomers joined via 1-4 and 1-6 glycosidic bonds to form highly branched chains.
The following features allow glycogen to function as a store of energy:
Insoluble - It does not affect the water potential of cells, and so water does not enter cells by osmosis.
Compact - A lot of glucose can be stored in a small space.
More highly branched than starch - Enzymes can easily hydrolyse the glycosidic bonds to rapidly release glucose.
Large - It cannot diffuse out of cells.
Hydrolysis releases alpha-glucose monomers - These are readily used in respiration.
Cellulose
Cellulose is a polysaccharide formed from beta-glucose. Its primary use is to provide structural support for plant cell walls.
Cellulose is made up of many beta-glucose monomers joined together via glycosidic bonds. However, if two beta-glucose monomers line up next to each other, the hydroxyl groups on carbon 1 and carbon 4 are too far from each other to react.
To fix this, every other beta-glucose molecule is inverted by 180° (flipped upside down). This brings the hydroxyl groups (OH) close enough together to react.
When many beta-glucose monomers join together they form long, straight, unbranched chains. The alternating inversion of the beta glucose molecules also allows for hydrogen bonds to form between individual chains. Although each hydrogen bond itself is relatively weak, the huge number of these bonds provides great strength to cellulose as a whole.
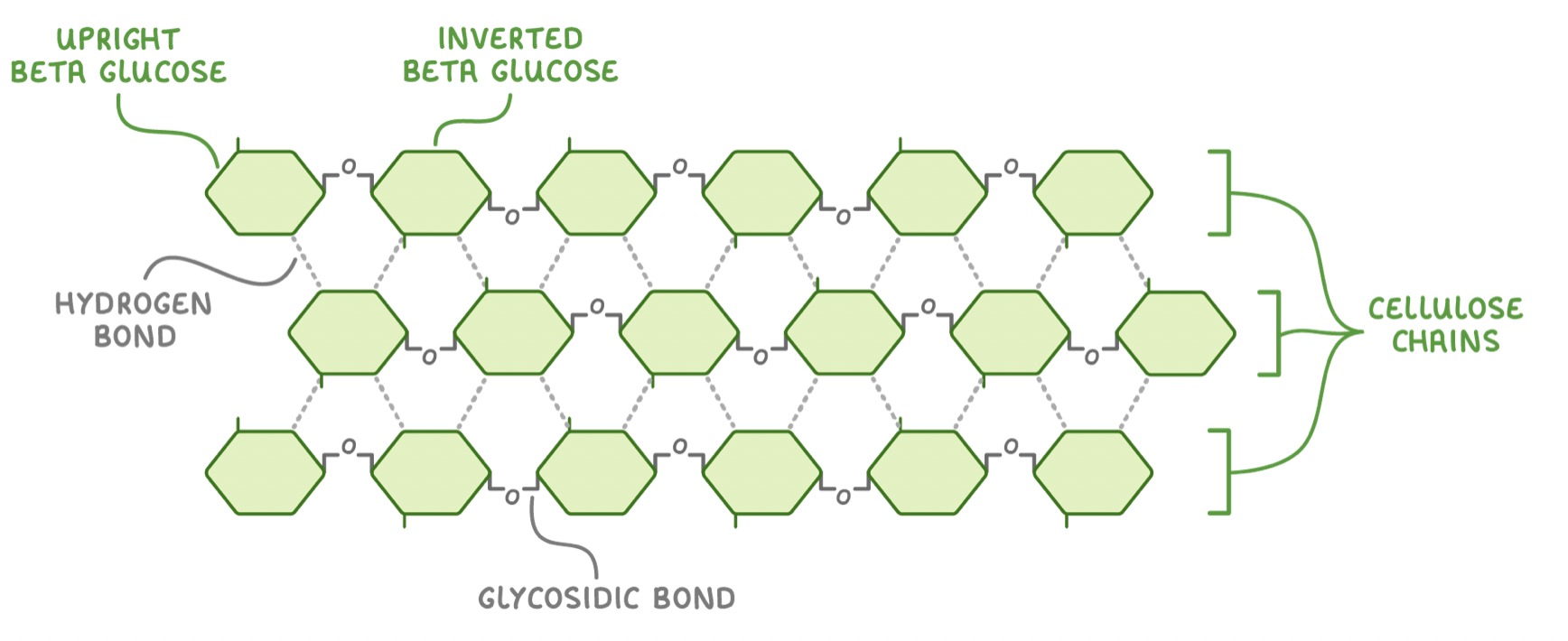
Macrofibrils and microfibrils
Multiple cellulose chains become tightly cross linked via hydrogen bonds to form bundles called microfibrils.
These microfibrils join together to make macrofibrils which combine to make strong cellulose fibres in the plant cell wall.
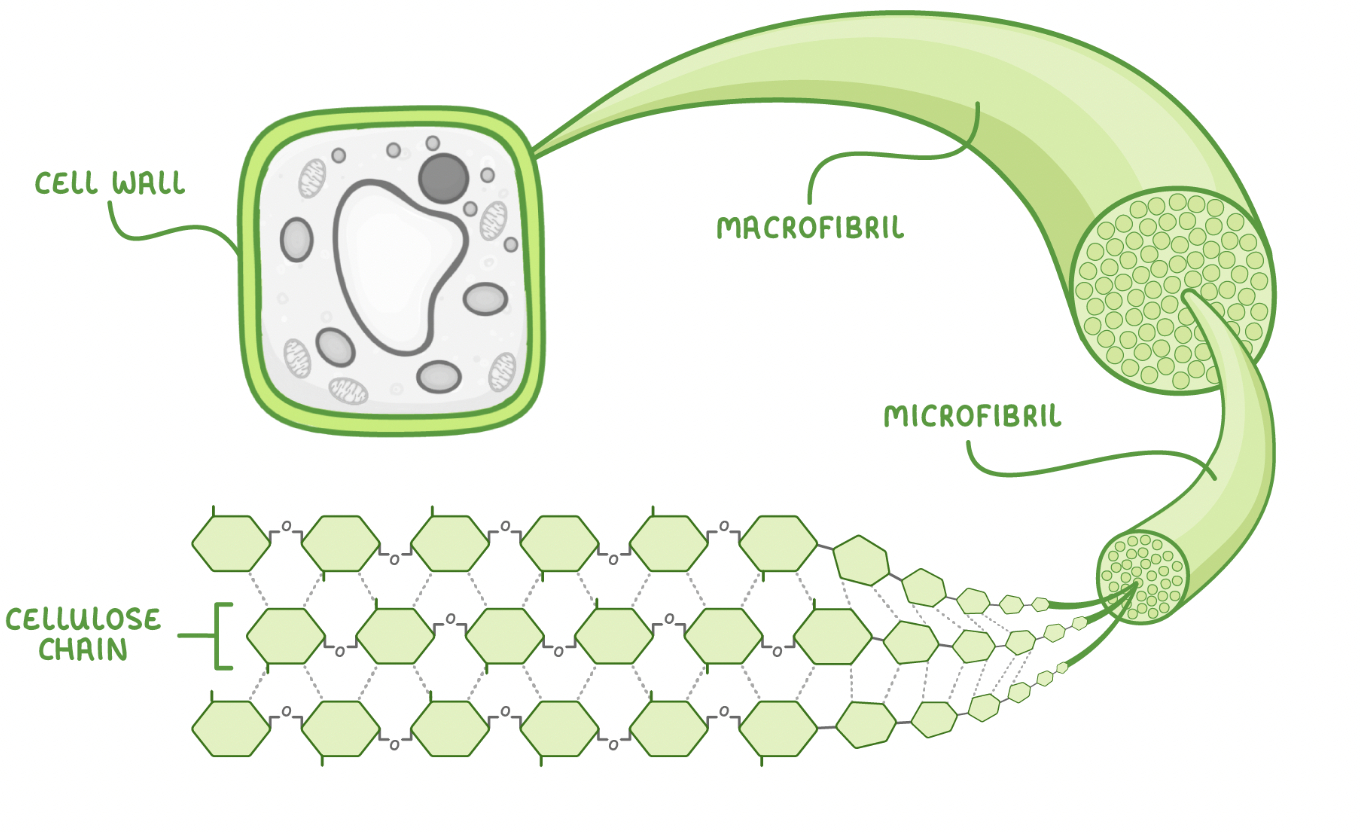
Adaotations of cellulose for its role
Long, straight, and unbranched chains - These provide rigidity to the cell wall.
Hydrogen bonds - These cross link the chains to add collective tensile strength.
Microfibrils - These provide additional strength.
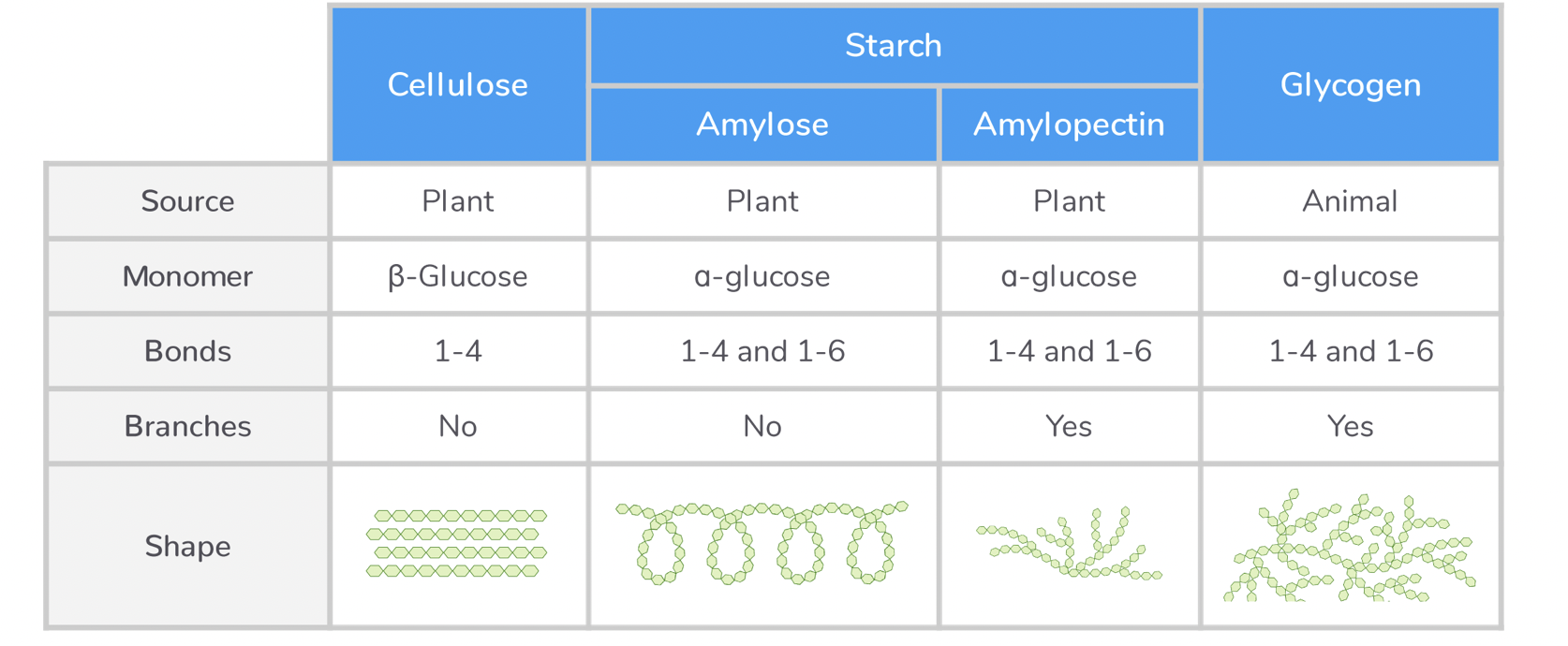
Comparing starch, glycogen and cellulose
2.7 Carbohydrates Tests
2.7 Carbohydrates Tests
Reducing and non reducing sugars
These categories are reducing sugars and non-reducing sugars:
Reducing sugars include all monosaccharides and some disaccharides such as maltose and lactose.
Non-reducing sugars include some disaccharides such as sucrose and all polysaccharides.
Testing for reducing sugars
Place 2 cm3 of your food sample into a test tube.
Add an equal volume of Benedict's reagent.
Heat the mixture in a gently boiling water bath for 5 minutes.
If a reducing sugar is present, the mixture will change from a blue solution to a brick red precipitate.
A positive result will form a brick red precipitate, however the colour seen is a mixture of the precipitate and the blue Benedict's reagent.
The concentration of reducing sugar determines the colour of this mixture:
Blue - This indicates no reducing sugar is present.
Green - This indicates a low concentration.
Orange - This indicates a medium concentration.
Brick-red - This indicates a high concentration.
This allows you to compare the concentration of reducing sugar between different samples.
Quantitative methods to determine the concentration of reducing sugars
Use a colorimeter to measure the absorbance of each solution.
Filter the solution and weigh the precipitate.
Testing for non-reducing sugars
Non-reducing sugars give a negative result (blue solution) for the normal reducing sugars test. To test for these types of sugars, you must first hydrolyse them into their monosaccharide components before we can do the rest of the test.
Steps to find out whether a sample contains a non-reducing sugar:
Carry out the test for reducing sugars, and if the result is negative (turns blue), continue with the next steps.
Add 2 cm3 of the food sample to 2 cm3 of dilute hydrochloric acid.
Heat the mixture in a gently boiling water bath for 5 minutes (the acid will hydrolyse disaccharides into monosaccharides).
Neutralise the mixture by adding sodium hydrogencarbonate solution.
Retest this mixture using the test for reducing sugars.
If non-reducing sugars were present at the start, the mixture will now change from a blue solution to a brick red precipitate.
Testing for starch
To find out if a sample contains starch, you must carry out the iodine test.
Steps to find out whether a sample contains starch:
Place 2 cm3 of your food sample into a test tube.
Add a couple of drops of iodine solution and shake.
If starch is present, the solution will turn from orange to blue-black.
2.8 Lipids introduction
2.8 Lipid introduction
What are lipids
Lipids are biological molecules that contain the elements carbon (C), hydrogen (H), and oxygen (O). However, lipids contain a much lower proportion of oxygen than carbohydrates.
Lipids are not made up of long chains of monomers, meaning they are not considered as polymers.
Role of lipids
The main functions of lipids:
Energy supply - Lipids can be oxidised to provide energy to cells.
Structural components - Phospholipids are used in cell membranes.
Waterproofing - Insoluble lipids are used to form water-resistant barriers.
Insulation - Lipids can help retain heat or act as electrical insulators.
Protection - Delicate organs are surrounded by a layer of fat.
Fatty acids
Most lipids are made up of fatty acids combined with an alcohol (usually glycerol).
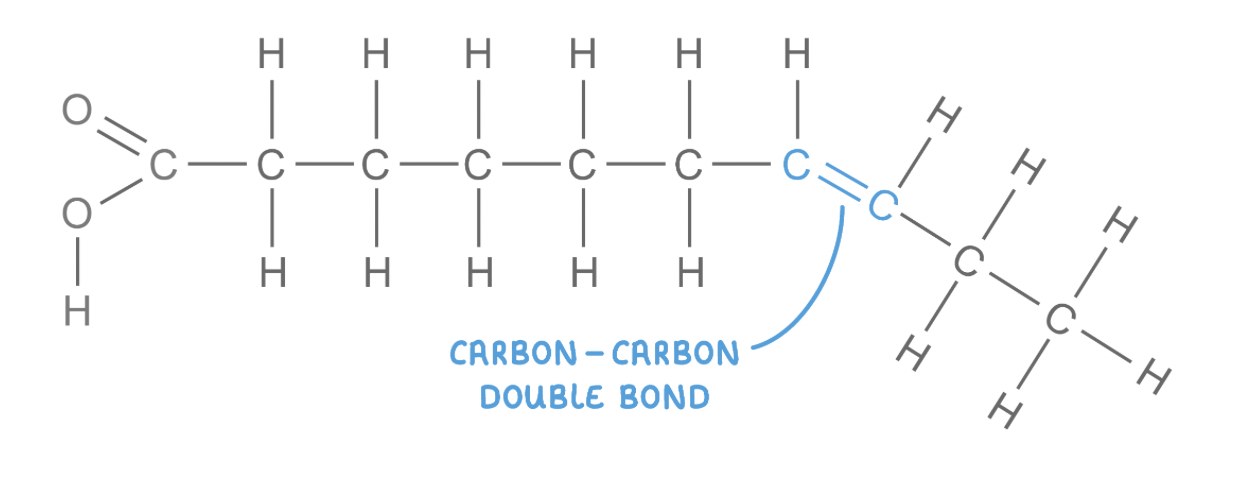
Fatty acids consist of a carboxyl group (-COOH) attached to a hydrocarbon chain (R group).
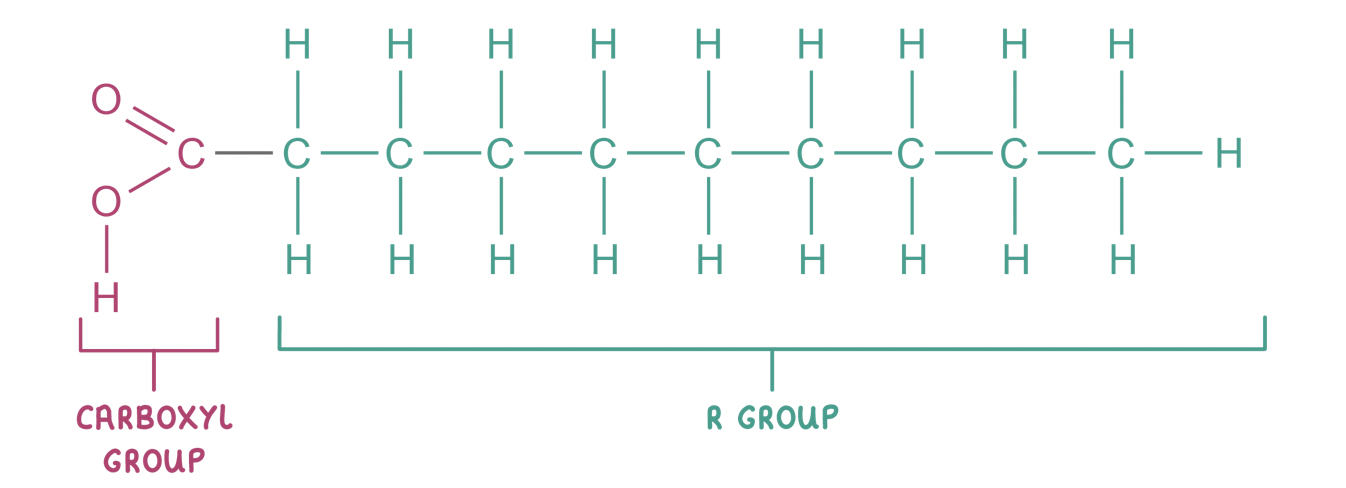
Saturated fatty acids
These have hydrocarbon chains that are 'saturated' with hydrogen, meaning all carbon atoms are bonded to the maximum number of hydrogen atoms.
The hydrocarbon chain has no carbon-carbon double bonds.
Lipids that contain saturated fatty acids have higher melting points and so are usually solid at room temperature (fats).
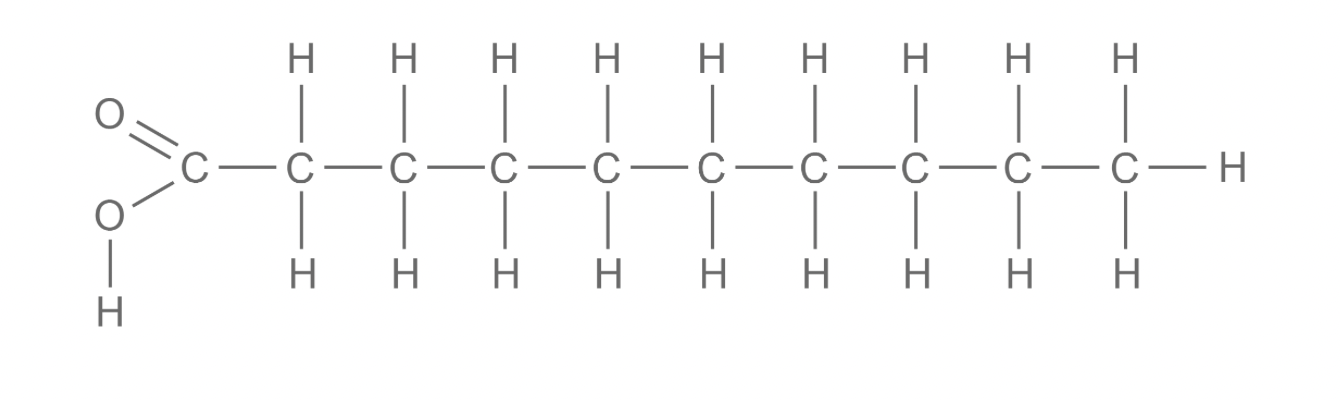
Unsaturated fatty acids
These have hydrocarbon chains that do not contain the maximum number of hydrogen atoms bonded to the carbon atoms.
The hydrocarbon chain has at least one carbon-carbon double bond, which causes the chain to kink.
Lipids that contain unsaturated fatty acids have lower melting points and so are usually liquid at room temperature (oils).
Unsaturated fatty acids may either be monounsaturated or polyunsaturated:
Monounsaturated - One double bond.
Polyunsaturated - Two or more double bonds.
Testing for lipids
Emulsion test-
Steps to find out whether a sample contains lipids:
Place your food sample in a test tube.
Add 2 cm3 of ethanol.
Shake.
Add 2 cm3 of distilled water.
If lipids are present, a milky white emulsion will appear.
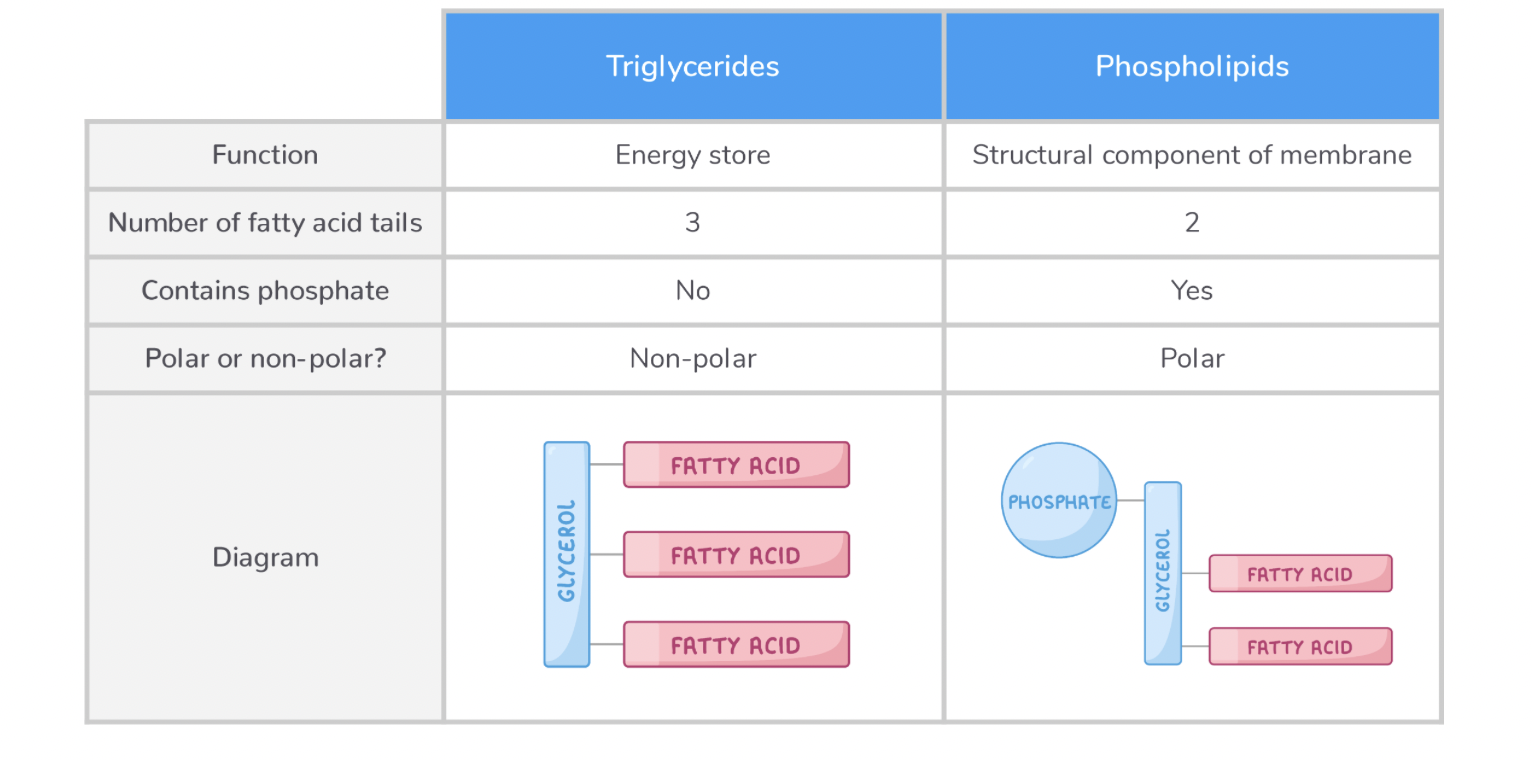
2.9 Lipids: Triglycerides, Phospholipids & Cholesterol
2.9 Lipids: Triglycerides, Phospholipids & Cholesterol
Triglycerides
A triglyceride is a type of lipid used as a store of energy in animals, plants, and some bacteria.
A triglyceride consists of a glycerol backbone attached to three fatty acid tails. Each fatty acid tail contains a hydrocarbon chain (R) which can vary in length and may be saturated or unsaturated.
Features that allow triglycerides to store energy efficiently:
Long hydrocarbon tails - Their many carbon-hydrogen bonds can be broken to release energy.
Low mass to energy ratio - Lots of energy can be stored in a small volume.
Insoluble - They do not affect the water potential of cells as they are large and non-polar.
High ratio of hydrogen to oxygen atoms - Triglycerides will release water when oxidised.
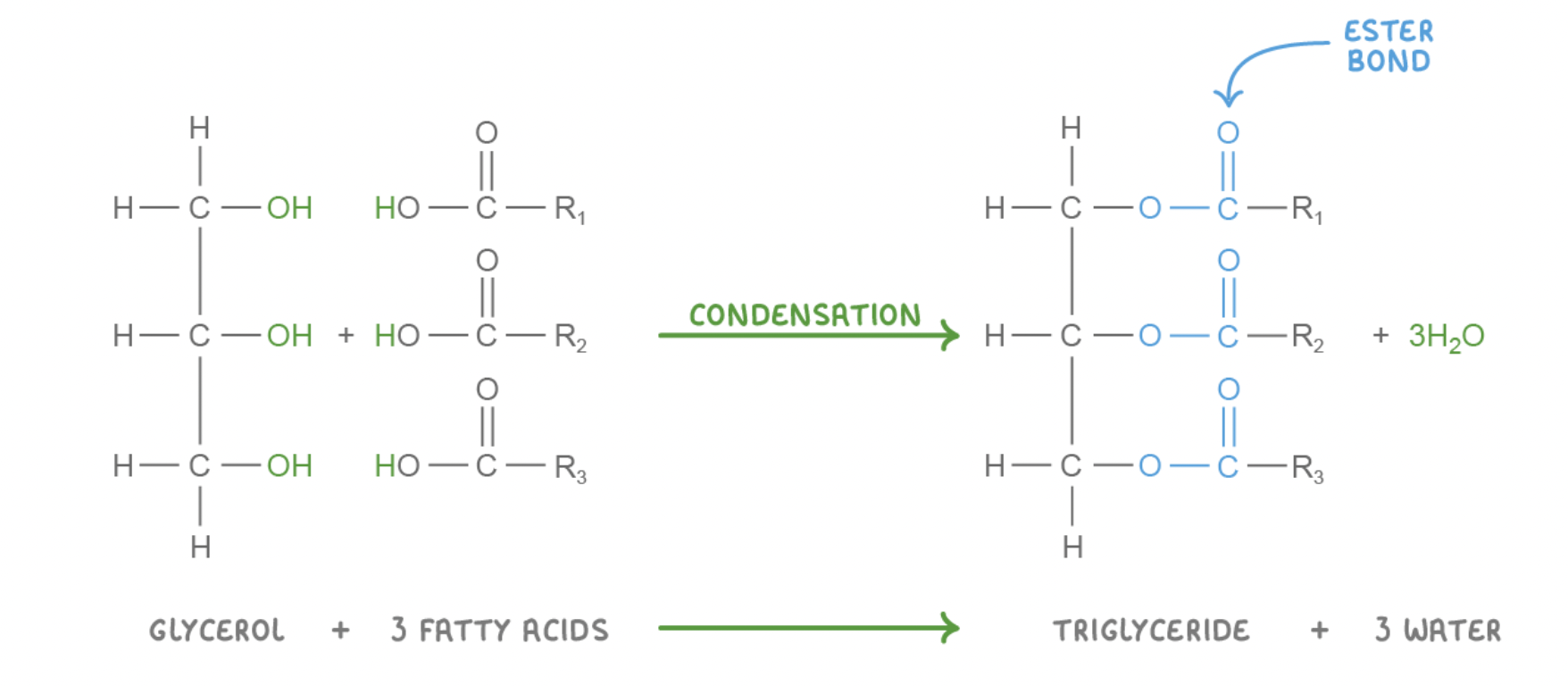
Triglyceride formation and breakdown
Triglycerides are synthesised via condensation reactions and broken down via hydrolysis reactions. These reactions involve the formation or the breakdown of covalent bonds known as ester bonds.
Condensation reaction with triglycerides
The hydroxyl groups (OH) on the glycerol and on the three fatty acids react together to release three water molecules (H2O).
This results in three ester bonds between the glycerol and the fatty acids.
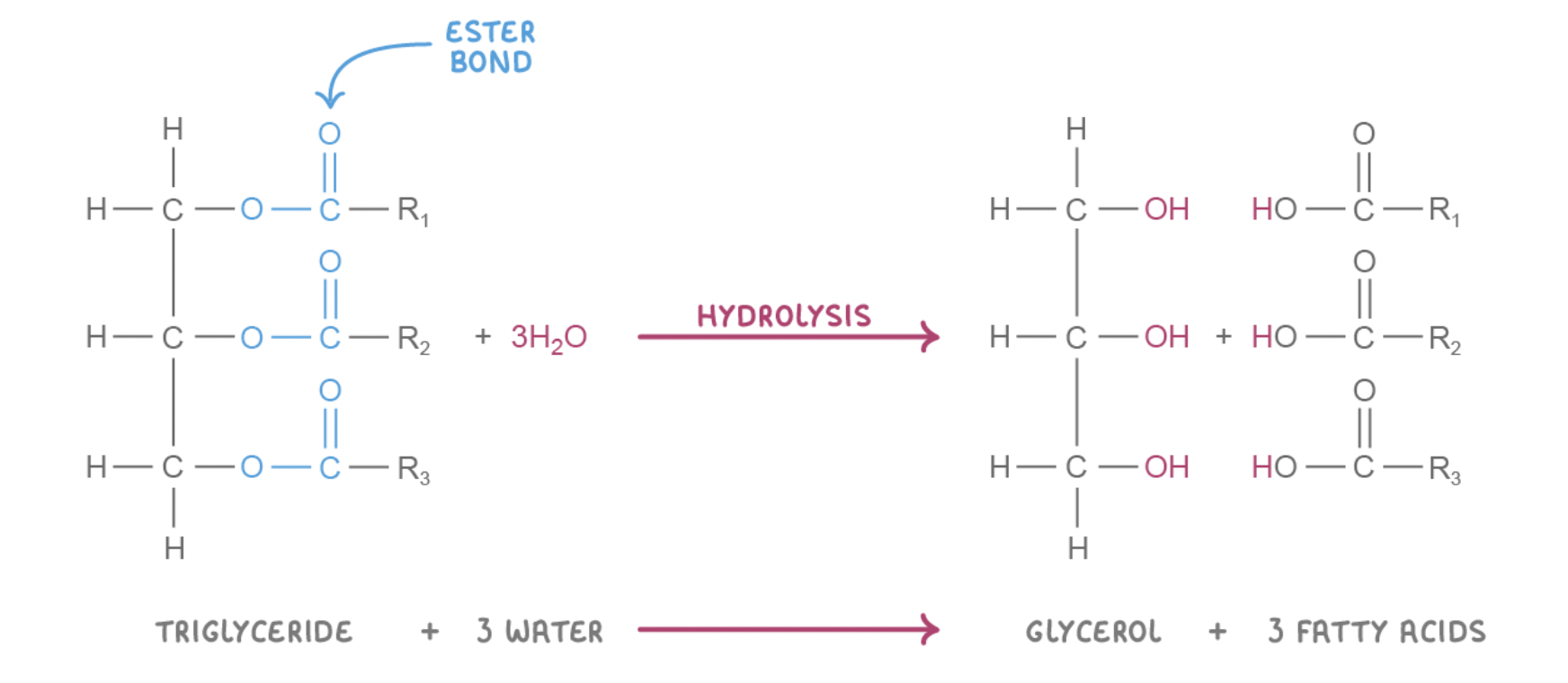
Hydrolysis reaction with triglycerides
The addition of three water molecules (H2O) breaks the ester bonds.
This separates the glycerol and the fatty acids.
Phospholipid
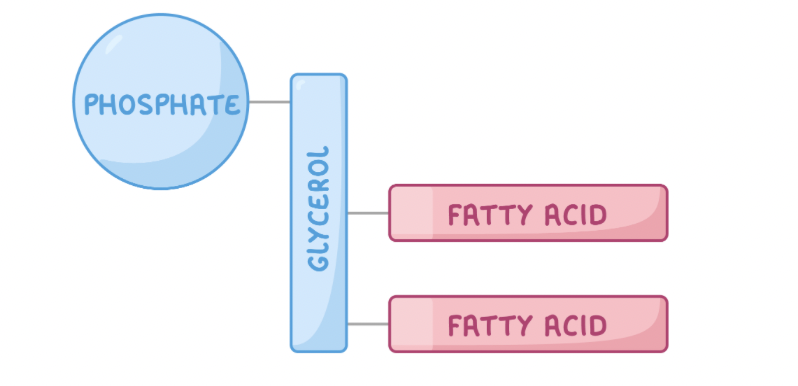
A phospholipid is a type of lipid used as a structural component of the cell membrane.
They are similar to triglycerides except one of the fatty acid tails is replaced by a phosphate group.
A phospholipid is made up of two parts:
A hydrophilic 'head' - This contains glycerol and phosphate.
A hydrophobic 'tail' - This contains fatty acids.
So they are Polar.
When phospholipids are placed in water, they arrange themselves into a double layer (bilayer) so that the hydrophilic heads are facing out (towards the water) and the hydrophobic tails are facing in (away from the water). This arrangement creates a hydrophobic centre in the bilayer so that water-soluble substances cannot pass through.
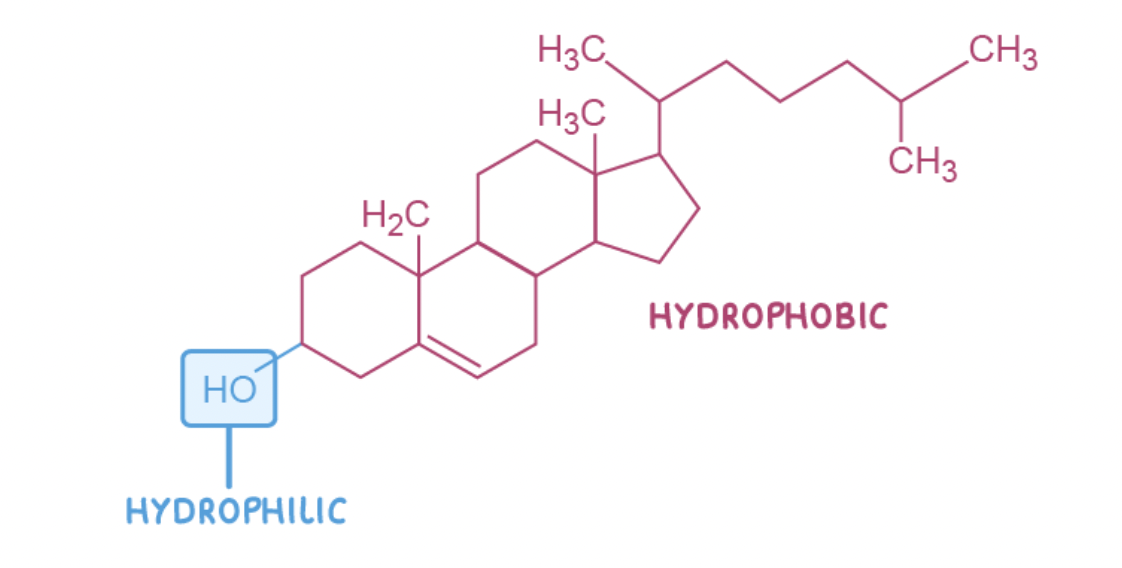
Cholesterol
Cholesterol is a type of lipid known as a sterol and is used by animal cells to increase the stability of the cell membrane.
Cholesterol is also used to make vitamin D, steroid hormones, and bile.
Like phospholipids, cholesterol is a polar molecule. The hydroxyl group (OH) is hydrophilic whereas the rest of the molecule is hydrophobic
Cholesterol binds to phospholipid fatty acid tails, causing the phospholipids to pack more closely together. This reduces the fluidity of the cell membrane
Comparing triglycerides and phospholipids