Entropy
0.0(0)
0.0(0)
Card Sorting
1/5
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
1
New cards
What is entropy?
Entropy is the measure of disorder in a system.
2
New cards
The more disordered a system is, the…
Higher the level of entropy.
3
New cards
Which has the greatest level of entropy: solids, liquids or gases?
Gases.
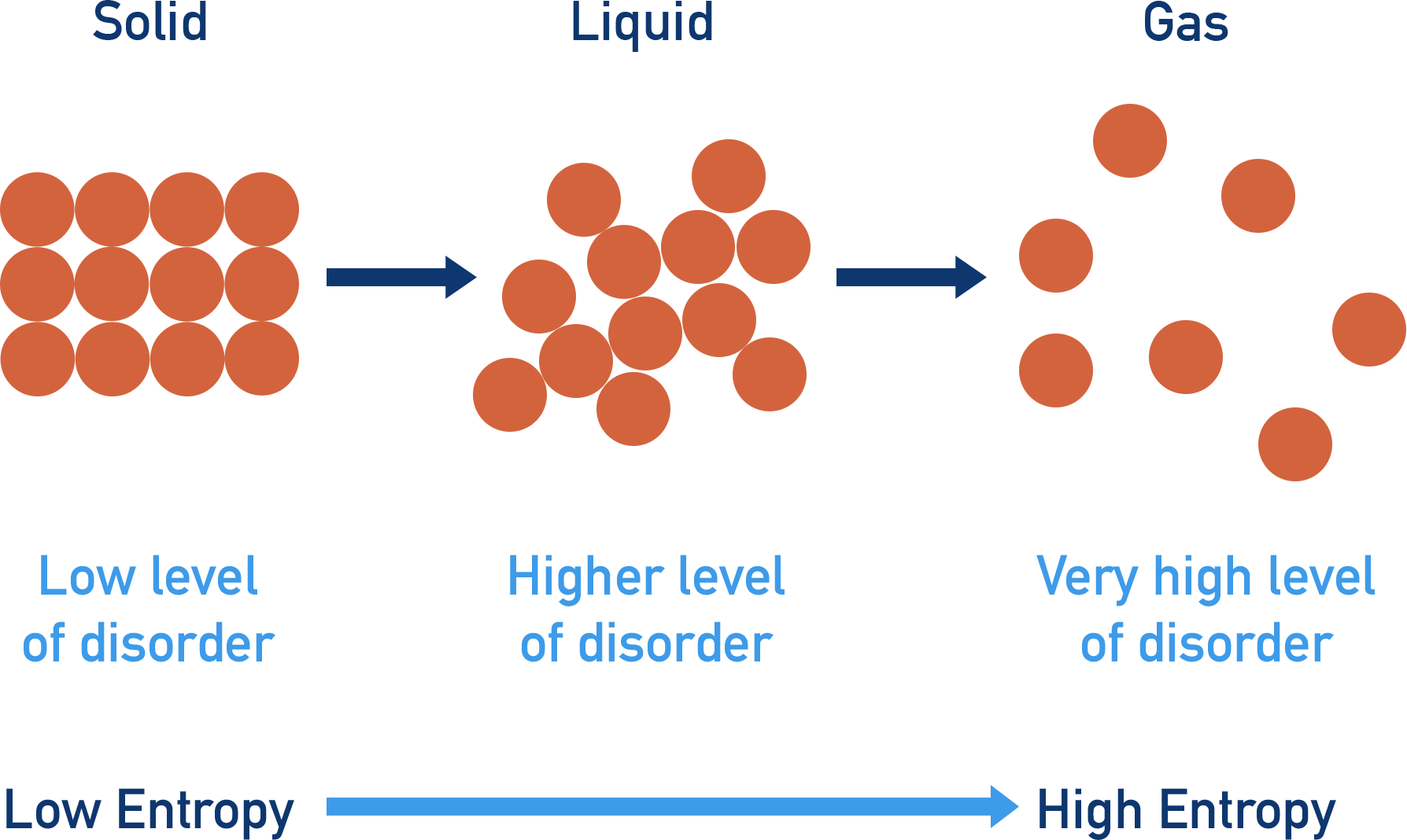
4
New cards
How does the number of particles affect the entropy change?
If a reaction is in the same state but more moles are produced, the entropy increases.
5
New cards
How is the entropy change calculated?
ΔSΘ = ΣSΘ(products) – ΣSΘ(reactants)
6
New cards
What are the units for entropy change, ΔS?
J K-1 mol-1