Topic 2: Bonding angles
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
What is special about bigger atoms about bigger atoms, allowing to form more bonding pairs?
Bigger atoms can expand their octet
How is the shape of a molecule determined?
The shape of a molecule is determine by the number of lone pairs, electron pairs arranged to minimise repulsion. The higher the number of lone pairs → the greater the lone pair - bond pair repulsion than bond pair- bond pair repulsion → and the more the bonding angle is reduced.
How do you calculate number of lone pairs on a bonded atom and how do you calculate total number of electron pairs respectively?
Calculations:
Use 0.5(number of outer shell electrons on central atom - number of atoms bonded to central atom) to calculate number of lone pairs
Use bonding pairs + lone pair, to find out total electron pairs
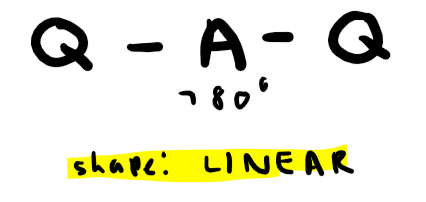
Electron pairs: 2 Number of bonding pairs: 2 Number of lone pairs: 0
Shape?
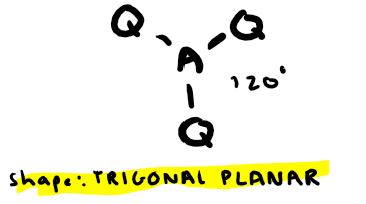
Electron pairs: 3 Number of bonding pairs: 3 Number of lone pairs: 0
Shape?
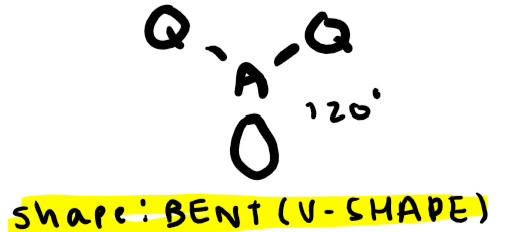
Electron pairs: 3 Number of bonding pairs: 2 Number of lone pairs: 1
Shape?
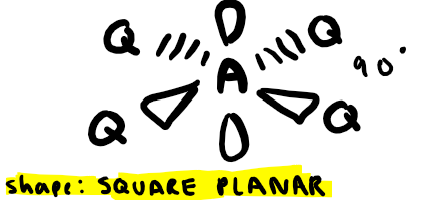
Electron pairs: 4 Number of bonding pairs: 4 Number of lone pairs: 0
Shape?
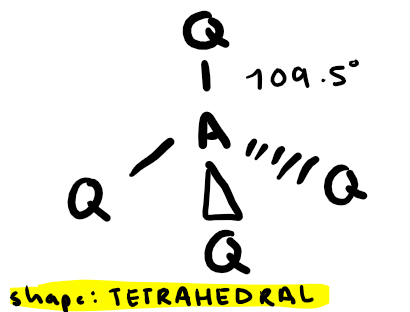
Electron pairs: 4 Number of bonding pairs: 3 Number of lone pairs: 1
Shape?
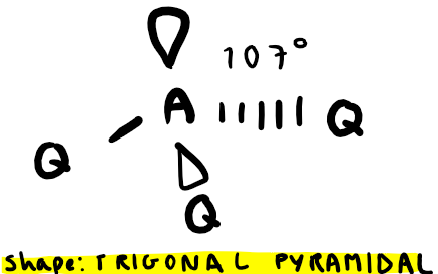
Electron pairs: 4 Number of bonding pairs: 2 Number of lone pairs: 2
Shape?
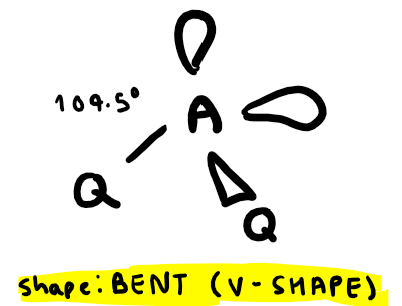
Electron pairs: 5 Number of bonding pairs: 5 Number of lone pairs: 0
Shape?
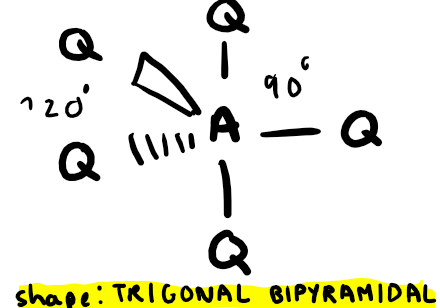
Electron pairs: 5 Number of bonding pairs: 4 Number of lone pairs: 1
Shape?
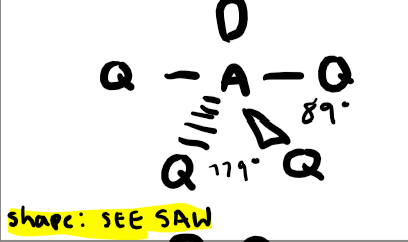
Electron pairs: 5 Number of bonding pairs: 3 Number of lone pairs: 2
Shape?

Electron pairs: 6 Number of bonding pairs: 6 Number of lone pairs: 0
Shape?
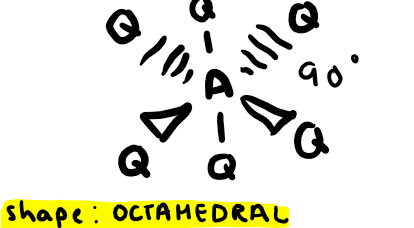
Electron pairs: 6 Number of bonding pairs: 5 Number of lone pairs: 1
Shape?
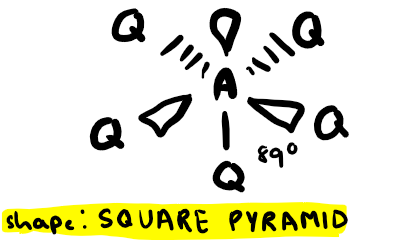
Electron pairs: 6 Number of bonding pairs: 4 Number of lone pairs: 2
Shape?