Separate Chemistry 1
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
47 Terms
What are the physical properties of transition metals?
High melting-point (except for mercury which is a liquid at room temperature)
High density
Stronger and harder than group 1 and 2 metals so they are often more suitable in construction materials
What are the chemical properties of transition metals?
Formation of coloured compounds
Catalytic activity
What are catalysts?
Substances that speed up the rate of reaction without:
Altering the products of a reaction
Changing chemically
Changing in mass at the end of a reaction (They are never used up)
What are the physical properties of group 1 and 2 metals?
Relatively low melting point
Relatively low density
Formation of white or colourless compounds
Lack of catalytic activity
What is corrosion?
The oxidation of metals
What is the corrosion of iron and steel called?
Rusting
What is needed for rusting to occur?
oxygen and water
How can we prevent rust on iron or steel?
Painting an object
Using oil or grease
Coating with plastic
Coating with another metal
Storing item in a vacuum container (so oxygen can’t get to it)
Put the item in a container with a desiccant (which absorbs water vapour)
What is electroplating and how is it done?
A thin layer of an unreactive metal like silver or gold is deposited on the surface of a metal object, keeping air and water out
This is done through electrolysis where silver ions lose their electrons to become positive
They move throughout the electrolyte and are attracted to the negative cathode which would be the object to be plated
The silver ions gain electrons to become silver atoms and the object which was used as a cathode is now plated with silver
Why is electroplating used?
To improve appearance
To improve an item’s resistance to corrosion
What is an alloy?
A mixture of a metal with one or more other elements
Why is iron by itself not used for many purposes and what is it made into?
Because it is too soft and needs to be mixed with other elements to make alloys that are stronger. When mixed with carbon, iron makes a steel
What are the most common types of steel?
Carbon steels
Consist of iron with up to 2% carbon
Are harder and stronger than iron alone
What is stainless steel?
An iron alloy that resists corrosion
Used to make cutlery, washing machine drums and dishwashers
Stainless steel contains chromium which forms an invisible layer of chromium oxide and top, stopping oxygen and water from reaching the iron
What are some alloys and their uses?
Alloy | Main metal | Mixed with | Typical properties | Uses |
|---|---|---|---|---|
Carbon steel | Iron | Carbon | Hard, strong | Building, bridges, car |
Magnalium | Aluminium | Magnesium | Low density | Car and plane parts |
Jewellery gold | Gold | Copper | Resistant to corrosion | jewellery |
Brass | Copper | Zinc | Hard, resistant to corrosion, good electrical conductor | electrical plugs, coins, instruments |
Why are alloys stronger than normal metal atoms?
Normal metal atoms are arranged in neat rows, thins make it easy for them to slide over each other which is why they are malleable (can be bent)
Alloys have a mixture of a main metal and a different element, these different sized particles mean that it is harder for the rows of atoms to slide over each other so they are not malleable
What is the equation for concentration involving solutes and volume?
Concentration (Mol/dm³)= amount of solute (mol)/volume (dm³)
How can we convert between g/dm³ and mol/dm³?
mol/dm³→g/dm³ - multiply the concentration by the Mr
g/dm³→mol/dm³ - divide the concentration by the Mr
What is a theoretical yield?
The amount of product you can make in theory if no substance is lost
What is an actual yield?
The amount of mass you really get at the end of a reaction, this is always less than the theoretical yield
What is the equation for percentage yield?
percentage yield= (actual yield/theoretical yield) x 100
For what reasons may we not obtain the theoretical yield from a chemical reaction?
The reaction may not have finished or has reached equilibrium
There can be competing, unwanted reactions creating by-products
Losses during purification e.g. filtration
Liquid left behind after transferring containers
What is the equation for atom economy?
Atom economy= (Mr of desired products/Mr of all products) x 100
What is atom economy?
A way of measuring the number of atoms wasted when making a substance
How is 100% atom economy achieved?
When there is only one product
OR
When all the by-products are used as a feedstock for other processes
Why are reactions with high atom economies preferred in industrial processes?
It produces less waste so it is less harmful to the environment
Conserves limited resources
More sustainable
What does the volume occupied by a gas depend on?
The number of particles present
The temperature of the gas
The pressure of the gas
What is the molar volume?
The volume occupied by one mole of any gas at room temperature and atmospheric pressure
What is the value for molar gas volumes?
24 dm³/mol or 24,000 cm³/mol (This will be given to you if you need them for calculations)
What is the equation for the volume of a gas involving molar volume and moles?
Molar volume x moles
What is the equation to calculate the amount of moles in a gas involving volume of gas and molar volume?
Amount= volume of gas/molar volume
What is the calculation for mass involving Mr and moles?
Mass= Mr x amount of moles
What are reversible reactions?
Reactions where the reactants form the products and the products can form the reactants again
What is dynamic equilibrium?
When the rate of forward reaction=the rate of backward reaction
The concentrations of the reacting substances do not change
The forward and backward reaction keep on going, they do not stop at equilibrium
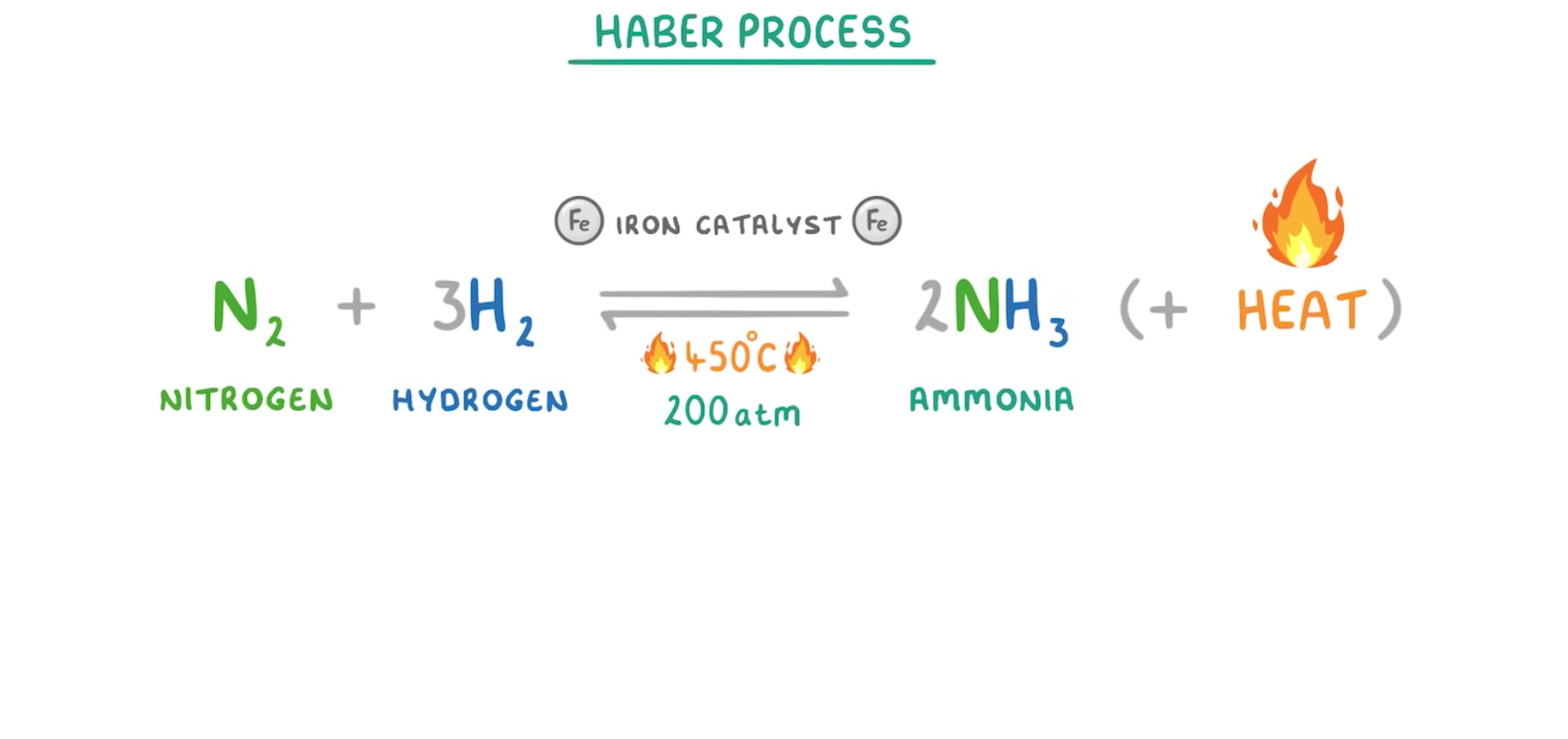
What is the Haber process?
The reaction of nitrogen and hydrogen to make ammonia
This reaction is exothermic
It is a reversible reaction- nitrogen and hydrogen from ammonia and ammonia breaks down to become nitrogen and hydrogen
See image
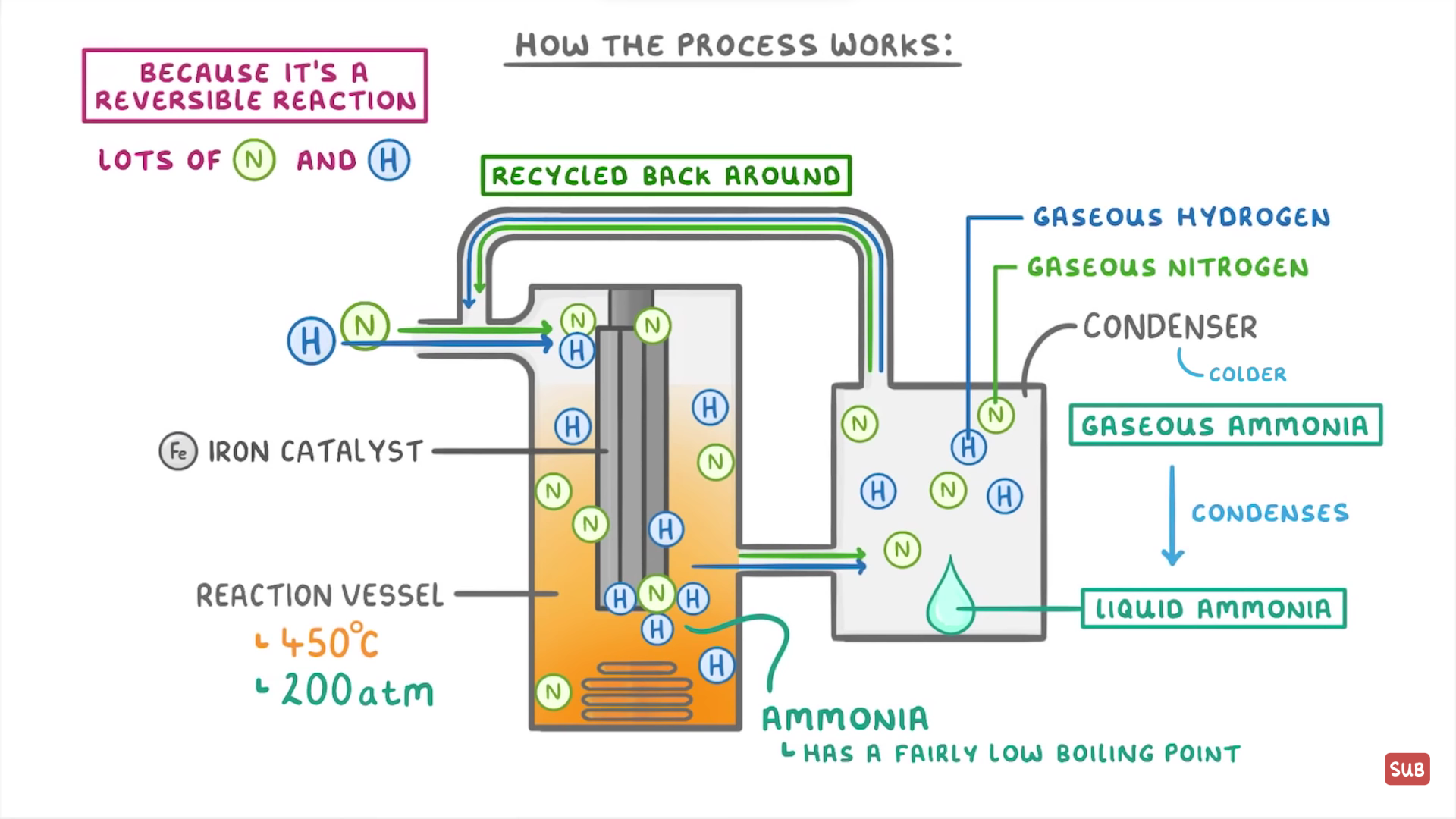
How does the Haber process work?
Nitrogen and hydrogen into the reaction vessel where they can mix together
The temp inside the vessel is 450 degrees celsius and the pressure is 200 atmospheres
There is an iron catalyst that speeds up the rate of reaction
Some of the N and H reacts to form ammonia but some N and H will still be in the vessel because it is a reversible reaction
The entire mixture is passed through a condenser and because ammonia has a low boiling point, it condenses into a liquid
The hydrogen and nitrogen in the condenser stay as gasses and are passed back into the reaction vessel so they are not wasted and this repeats the process again
See image
Why is a temperature of 450 degrees celsius used for the Haber process?
A lower temperature is needed to favour the forward reaction which gives a higher percentage yield
High temperatures are needed to increase the rate of reactions as the particles need a lot of kinetic energy
450 is used as a compromise because even though gives lower yield, it increases the rate of reaction
Generating heat is expensive
Why is a pressure of 200 atmospheres used for the Haber process?
A high pressure is needed for a higher percentage yield
A higher pressure favours the side with less molecules which would be the right side (ammonia)
High pressures increase the rate of reaction as it causes the particles to collide more frequently
Maintaining a high pressure is very expensive
High pressures can be very dangerous
How can changing the temperature, pressure, concentration of a reactant and adding a catalyst to an equilibrium reaction affect the rate of reaction?
Change in condition | Effect on equilibrium position | Effect on the rate of reacting equilibrium |
|---|---|---|
Temp increase | Moves in direction of endothermic reaction | Rate increased |
Pressure increase | Moves in direction of fewest molecules of gas | Rate increased (if reacting gases are present) |
Concentration of reactant increased | Moves in direction away from reactant | Rate increased |
Catalyst added | No change | Rate increased |
What are the conditions chose for industrial processes of equilibrium related to?
The availability of raw materials and energy supplies. Air and water are easily obtained but natural gas is difficult and expensive to obtain
The control of temperature and pressure. Many industrial processes are not allowed to reach equilibrium because this would take too long
The use of suitable catalyst such as iron. Processes can work at lower temps with catalysts, reducing cost and increasing yield
What are NPK fertilisers?
Fertilisers supplying the elements nitrogen, phosphorous and potassium
These elements must be supplied in soluble compounds:
Nitrogen- nitrate and ammonium salts
Phosphorous- phosphate salts
Potassium- potassium salts
How do chemical cells work with lead and bromine for example?
Negatively charged bromine ions lose their electrons and become neutral atoms
The electrons they lose are transported along the wire into the cathode
At the negative cathode positively charged lead ions pick up those electrons and become neutral lead atoms
This is our flow around the circuit which produces electricity. This process will continue until all of the electrolyte is used up. When they are used up the battery is ‘flat’.
How do hydrogen-oxygen fuel cells work?
Be aware that the anode is negative and the cathode is positive, the opposite of how it is in electrolysis
The electrodes are made of porous carbon which contains tiny holes
The electrodes also contains catalysts to speed up the rate of reaction
Hydrogen atoms come in through the side with the anode and lose their electrons, becoming hydrogen ions
The electrons move through the wire and to the cathode
The hydrogen ions then move towards the cathode through the electrolyte
Oxygen comes in from the side with the cathode and combines with the hydrogen and electrons to become water molecules
The water molecules leave the cell though the outlet
The movement of electrons from the anode to the cathode creates an electrical current
What happens when hydrogen gets to the anode in a hydrogen oxygen fuel cell?
Hydrogen is oxidised
This sets up a potential difference across the two electrodes
This is what drives the electrons around the circuit
What are the advantages and disadvantages of fuel cells?
Suitable for portable appliances
Cheap to manufacture
May contain harmful substances
Does not produce a voltage when one of the reactants is used up
What are the advantages of hydrogen-oxygen fuel cells?
Produces water as it’s only waste
Produces a voltage as long as the fuel and air are supplied
Lasts longer than batteries because they have simple designs
Less polluting to dispose of
What are the disadvantages of hydrogen-oxygen fuel cells?
Not suitable for portable appliances
Expensive to manufacture
Hydrogen is a gas meaning it takes more space to store than fossil fuels or batteries
Hydrogen is explosive when mixed with air- dangerous to store
To make the hydrogen fuel it requires energy, normally from fossil fuels