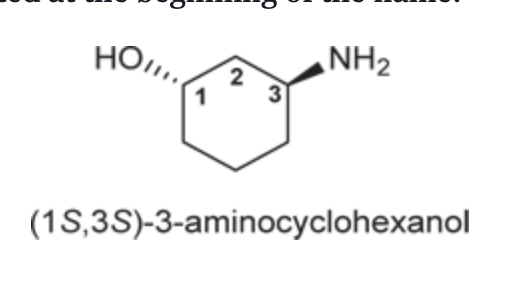
IUPAC Terms
1/117
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
118 Terms
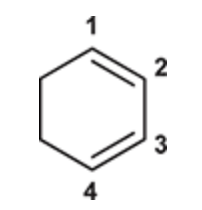
(Ch. 16) Draw the structure of 1,4-Cyclohexadiene
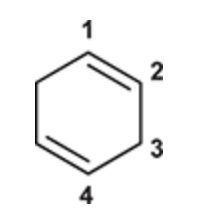
(Ch. 16) Draw the structure of 1,3-Cyclohexadiene
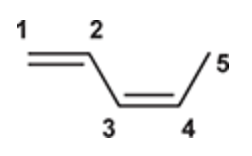
(Ch. 16) Draw the structure of (Z)-1,3-Pentadiene
(Ch. 16) Draw the structure of (2Z,4E)-Hepta-2,4-diene
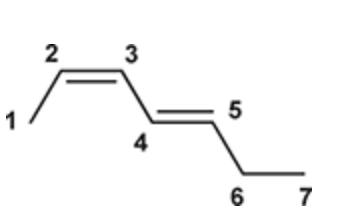
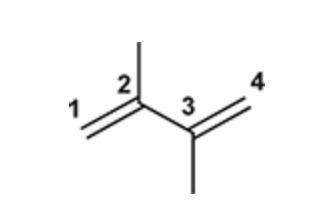
(Ch. 16) Draw the structure of 2,3-Dimethyl-1,3-butadiene
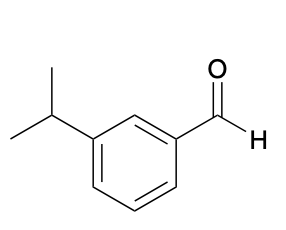
(Ch. 17) Provide a systematic name for the following
3-isopropylbenzaldehyde
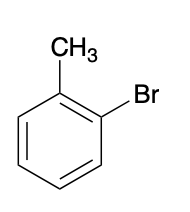
(Ch. 17) Provide a systematic name for the following
2-bromotoluene
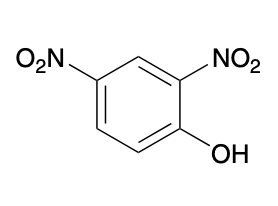
(Ch. 17) Provide a systematic name for the following
2,4-dinitrophenol
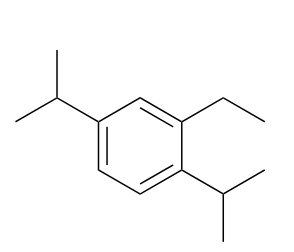
(Ch. 17) Provide a systematic name for the following
2-ethyl-1,4-diisopropylbenzene
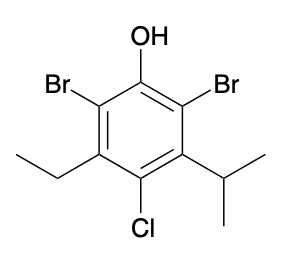
(Ch. 17) Provide a systematic name for the following
2,6-dibromo-4-chloro-3-ethyl-5-isopropylphenol
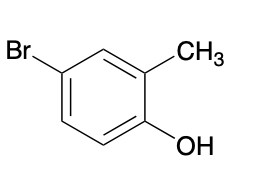
(Ch. 17) Aromatic compounds often have multiple names that are accepted by IUPAC. Provide three different systematic names for the following compound.
This gives the following three possible names:
4-bromo-2-methylphenol
5-bromo-2-hydroxytoluene
4-bromo-1-hydroxy-2-methylbenzene
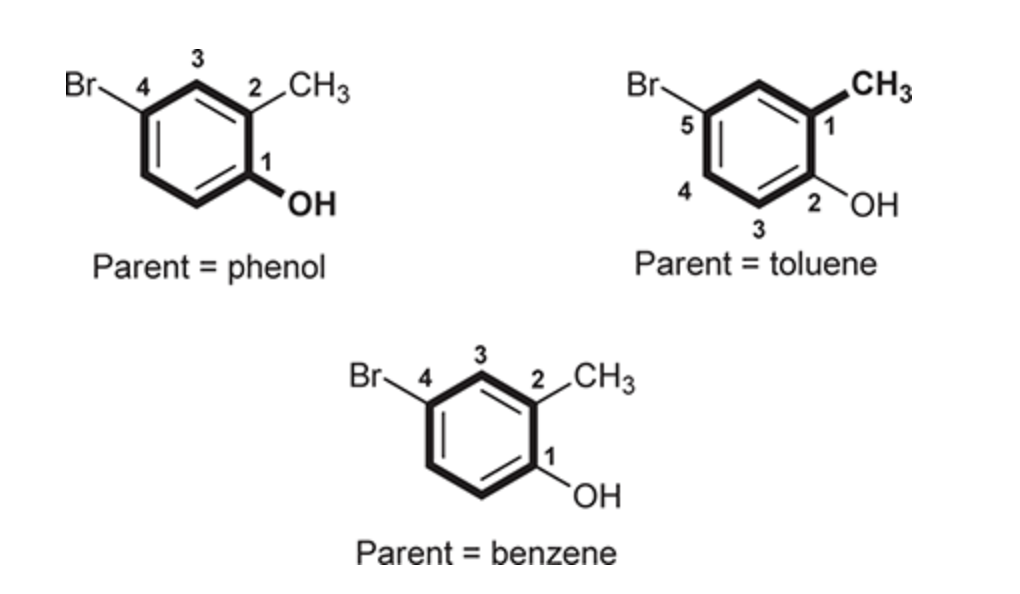
(Ch. 17) Draw the structure for the following compound: 2,6-Dibromo-4-chloroanisole
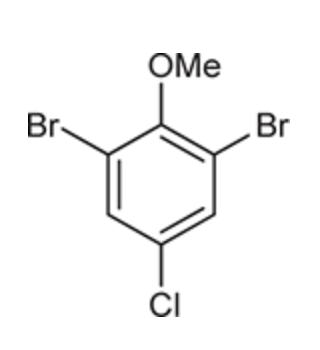
(Ch. 17) Draw the structure for the following compound: meta-Nitrophenol
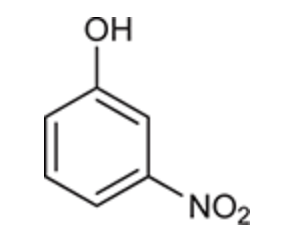
(Ch. 17) Provide at least five different acceptable names for the following compound.
meta-xylene
meta-dimethylbenzene
1,3-dimethylbenzene
meta-methyltoluene
3-methyltoluene
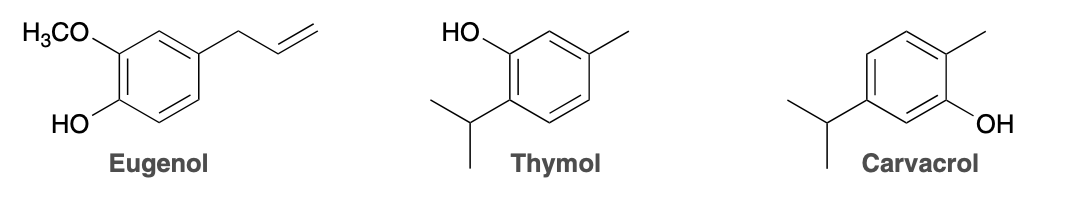
(Ch. 17) Aromatic compounds are commonly found in plant oils. For example, an extract of rosemary, which shows anti-oxidant activity, was found to contain the compounds eugenol, thymol, and carvacrol.These names are common names; however, each of these compounds also has more than one possible systematic name.
provide 3 systematic names for. eugenol and three for thymol (the hydrocarbon substituent in eugenol (-CH2-CH=CH2 ) is called an allyl group)
Eugenol:
As a benzene derivative: 4-allyl-1-hydroxy-2-methoxybenzene
As a phenol derivative: 4-allyl-2-methoxyphenol
As an anisole derivative: 5-allyl-2-hydroxyanisole
Thymol:
As a benzene derivative: 2-hydroxy-1-isopropyl-4-methylbenzene
As a phenol derivative: 2-isopropyl-5-methylphenol
As a toluene derivative: 3-hydroxy-4-isopropyltoluene
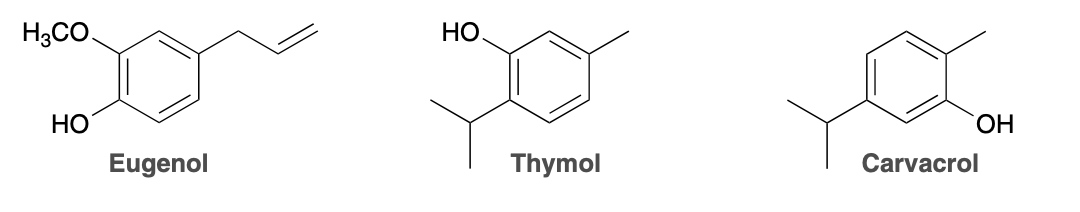
(Ch. 17) Aromatic compounds are commonly found in plant oils. For example, an extract of rosemary, which shows anti-oxidant activity, was found to contain the compounds eugenol, thymol, and carvacrol.These names are common names; however, each of these compounds also has more than one possible systematic name.
Provide at least one reason why 1-hydroxy-2-methyl-5-isopropylbenzene is an incorrect systematic name for carvacrol.
This name violates two of the rules for naming polysubstituted aromatic compounds. First, the substituents are not listed alphabetically. Second, if named with benzene as the parent, the methyl group needs to have the first locant in order to minimize the number of the third locant. Thus, a correct name would be 2-hydroxy-4-isopropyl-1-methylbenzene.
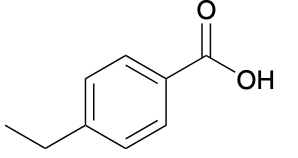
(Ch. 17) Provide a systematic name for the following compound
4-ethylbenzoic acid
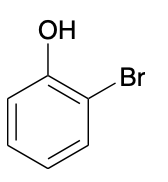
(Ch. 17) Provide a systematic name for the following compound
2-bromophenol
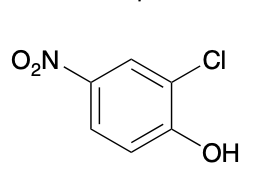
(Ch. 17) Provide a systematic name for the following compound
2-chloro-4-nitrophenol
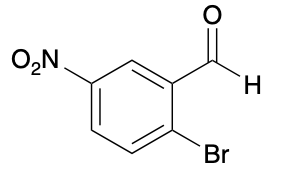
(Ch. 17) Provide a systematic name for the following compound
2-bromo-5-nitrobenzaldehyde
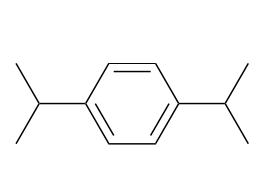
(Ch. 17) Provide a systematic name for the following compound
1,4-diisopropylbenzene
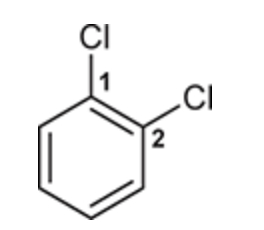
(Ch. 17) Draw a structure for each of the following compound: ortho-Dichlorobenzene
(Ch. 17) Draw a structure for each of the following compound: Anisole

(Ch. 17) Draw a structure for each of the following compound: meta-Nitrotoluene
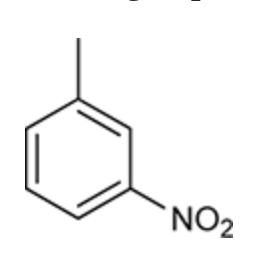
(Ch. 17) Draw a structure for each of the following compound: Aniline
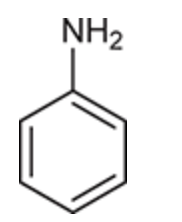
(Ch. 17) Draw a structure for each of the following compound: 2,4,6-Tribromophenol
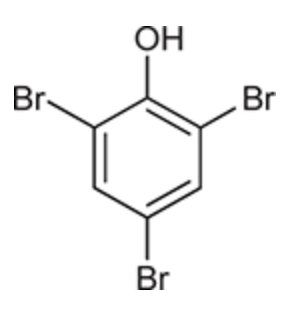
(Ch. 17) Draw a structure for each of the following compound: para-Xylene
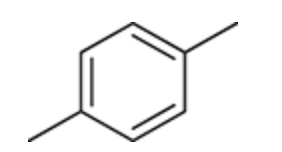
(Ch. 17) Draw structures for the eight constitutional isomers with the molecular formula C9H12 that contain a benzene ring.
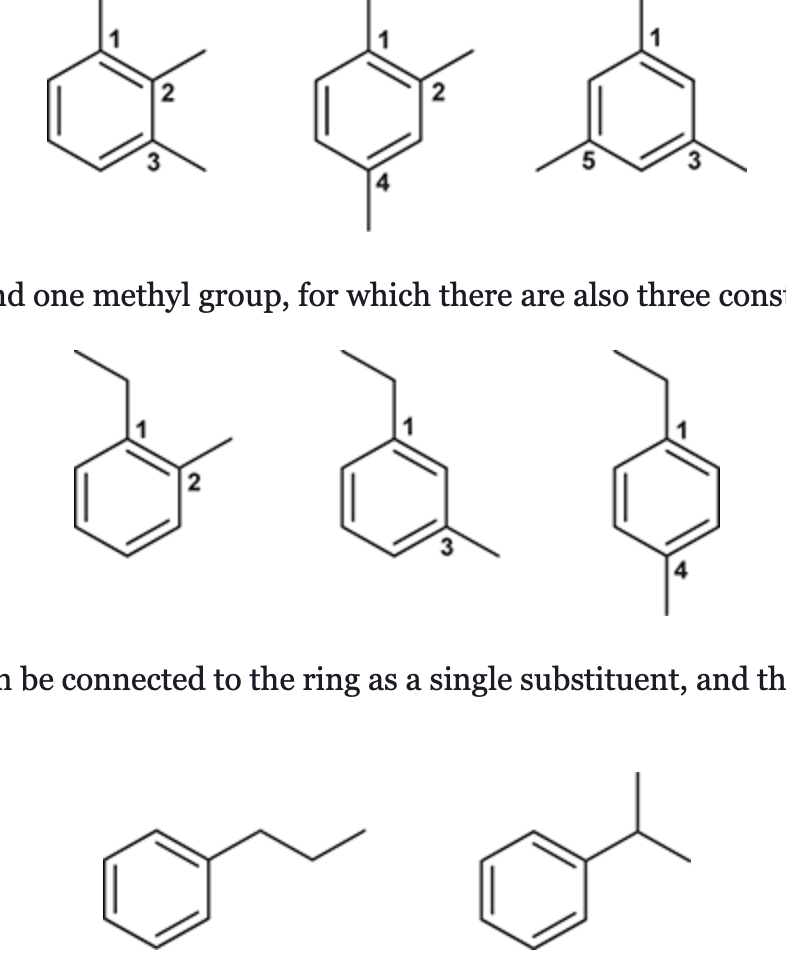
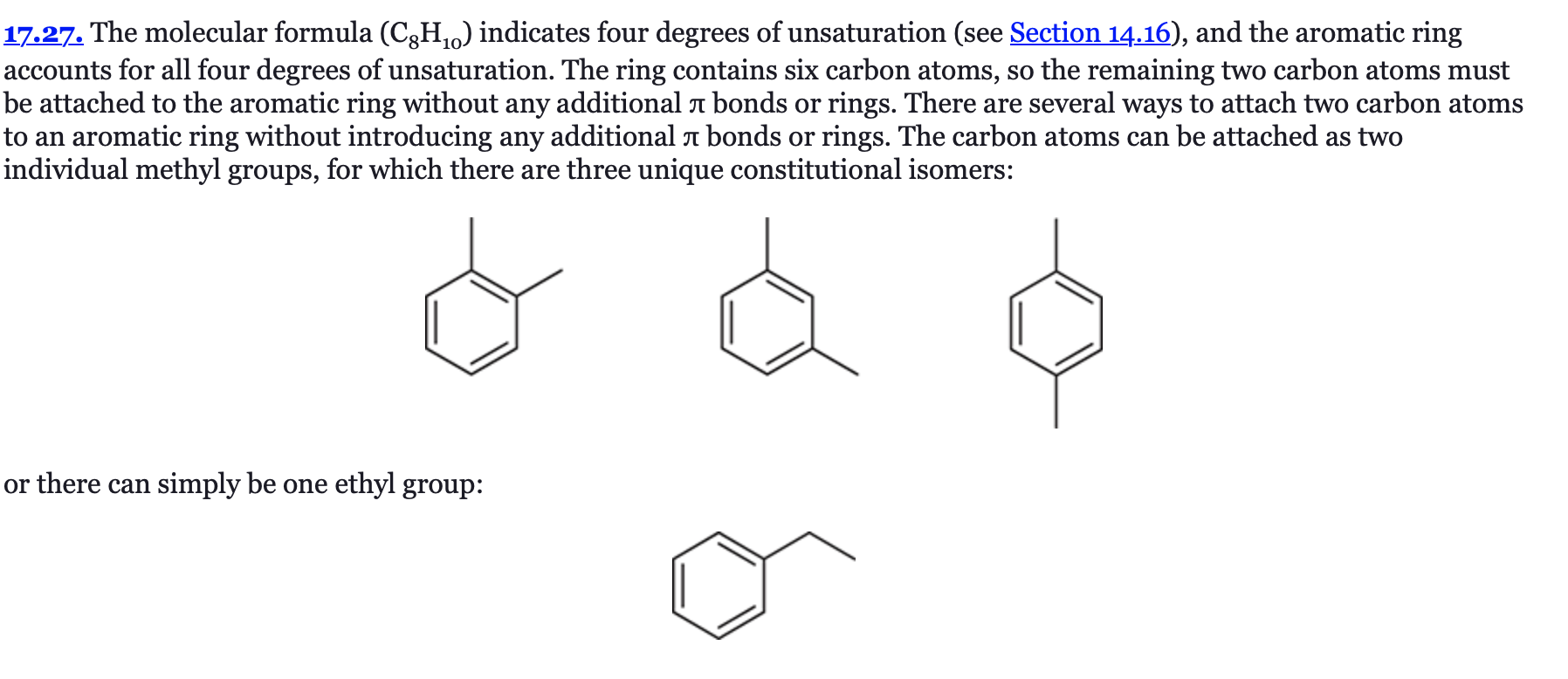
(Ch. 17) Draw structures for all constitutional isomers with the molecular formula C8H10 that contain an aromatic ring.
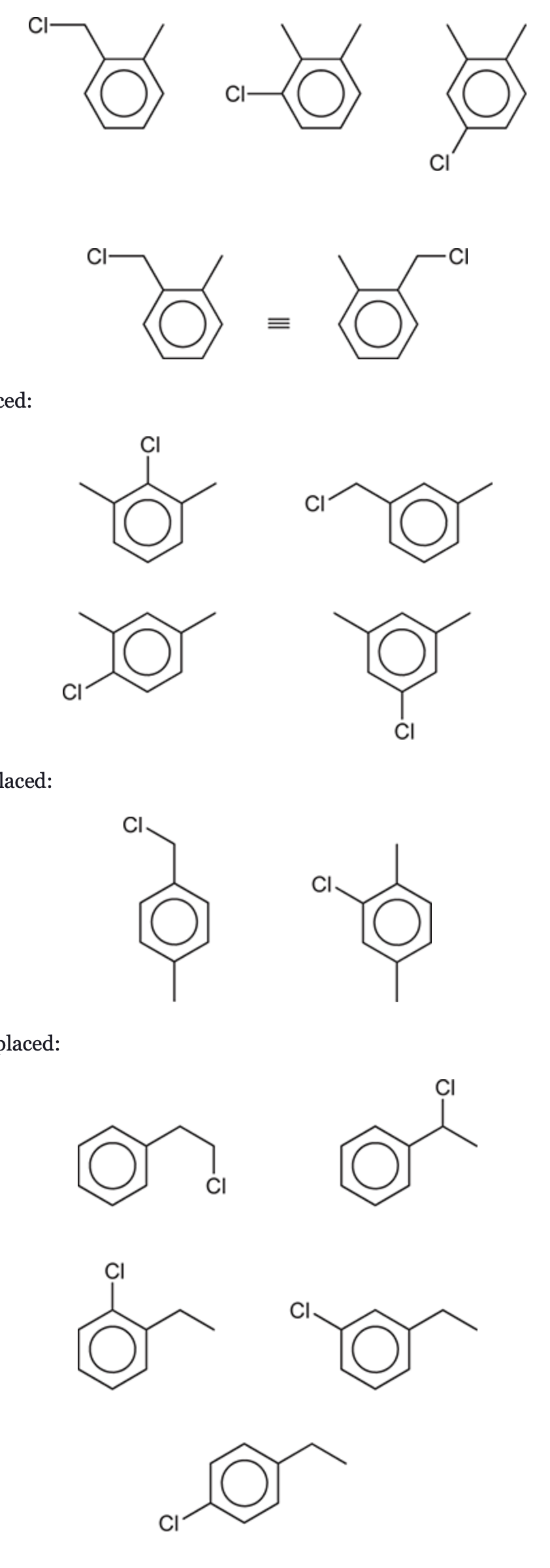
(Ch. 17) Draw all aromatic compounds that have the molecular formula C8H9Cl.
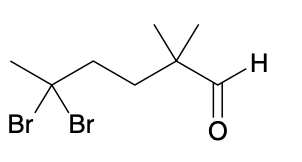
(Ch. 19) Assign IUPAC name to each of the following
5,5-dibromo-2,2-dimethylhexanal
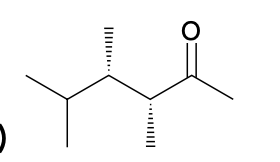
(Ch. 19) Assign IUPAC name to each of the following
(3R,4S)-3,4,5-trimeethyl-2-hexanone
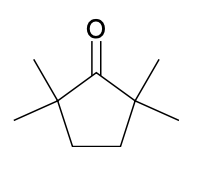
(Ch. 19) Assign IUPAC name to each of the following
2,2,5,5-tetramethylcyclopentanone
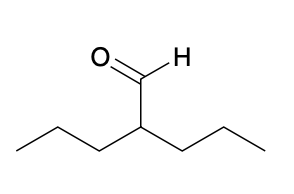
(Ch. 19) Assign IUPAC name to each of the following
2-propylpentanal
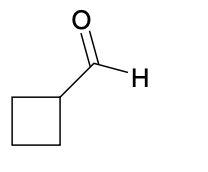
(Ch. 19) Assign IUPAC name to each of the following
cyclobutanecarbaldehyde
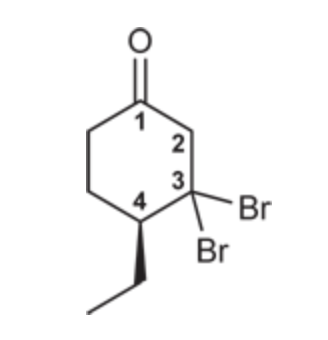
(Ch. 19) Draw the structure of the following compound: (S)-3,3-Dibromo-4-ethylcyclohexanone
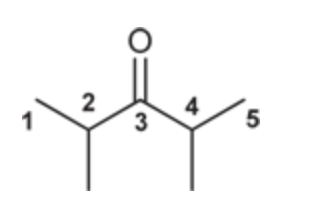
(Ch. 19) Draw the structure of the following compound: 2,4-Dimethyl-3-pentanone
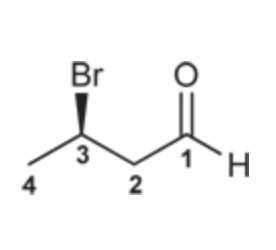
(Ch. 19) Draw the structure of the following compound: (R)-3-Bromobutanal
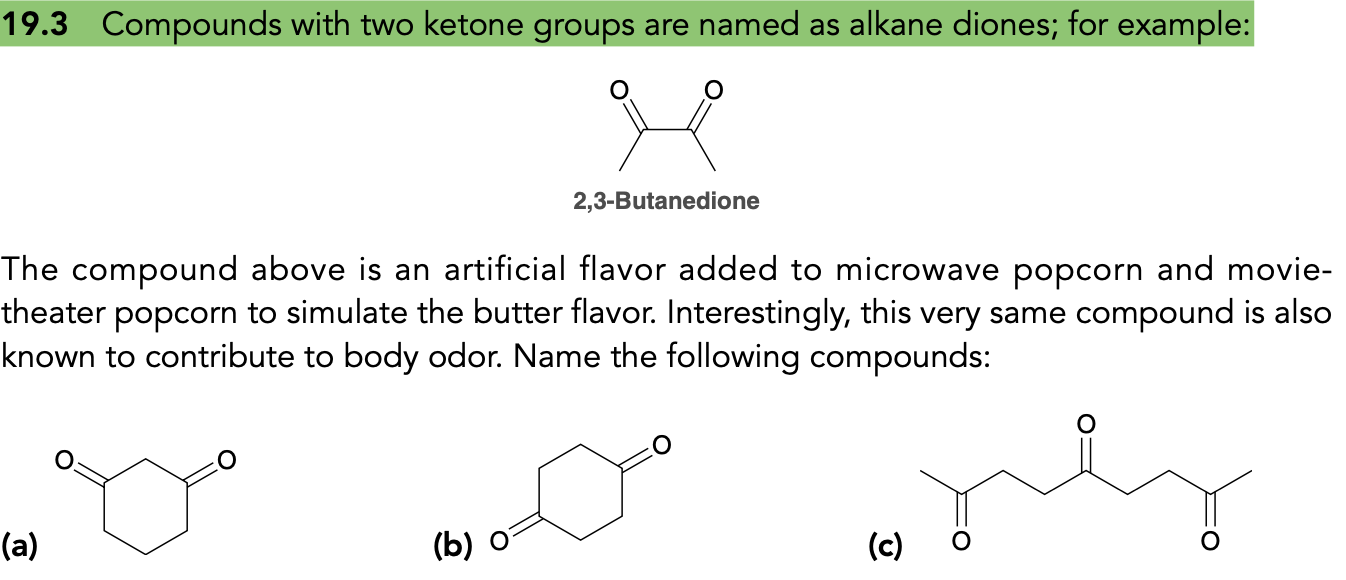
(Ch. 19)

(Ch. 19) Regular sunscreen use is an important part of keeping your skin healthy by protect- ing it from ultraviolet radiation, but some chemical agents in sunscreens may be harmful if absorbed into the skin. Research is ongoing to find less harmful alternatives and to under- stand the factors involved in skin absorption. The IUPAC names are given below for two common chemical sunscreens. Provide a structure for each:
(a) (2-Hydroxy-4-methoxyphenyl)-phenylmethanone (also known as oxybenzone).
(b) 1-(4-Methoxyphenyl)-3-[4-(1,1-dimethylethyl)phenyl]propane-1,3-dione
(also known as avobenzone).

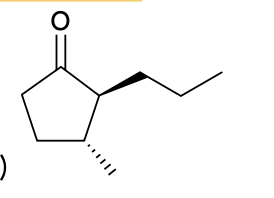
(Ch. 19) Provide IUPAC name for each
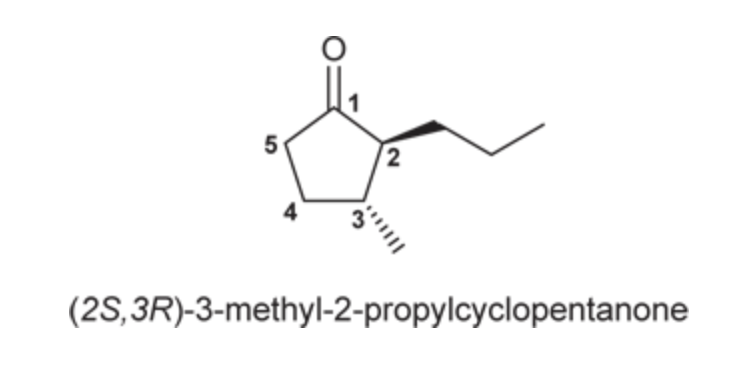
(Ch. 19) Provide IUPAC name for each
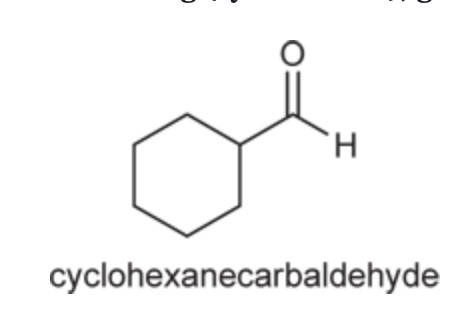
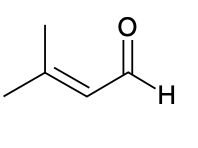
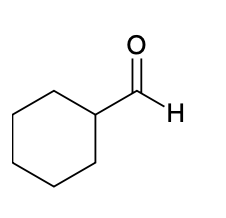
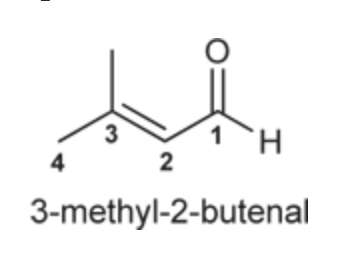
(Ch. 19) Provide IUPAC name for each
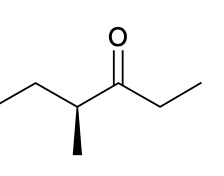
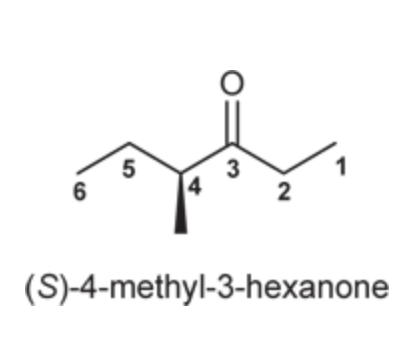
(Ch. 19) Provide IUPAC name for each
(Ch. 19) Draw the structure for each compound
Propanedial
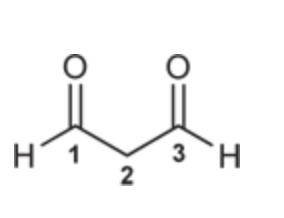
(Ch. 19) Draw the structure for each compound
4-Phenylbutanal
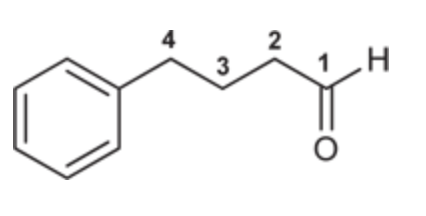
(Ch. 19) Draw the structure for each compound
(S)-3-Phenylbutanal
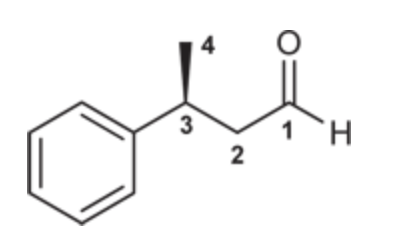
(Ch. 19) Draw the structure for each compound
3,3,5,5-Tetramethyl-4-heptanone
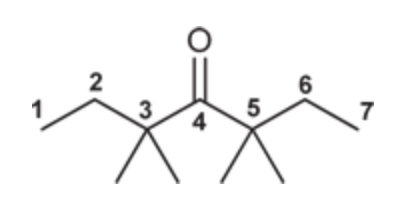
(Ch. 19) Draw the structure for each compound
(R )-3-Hydroxypentanal
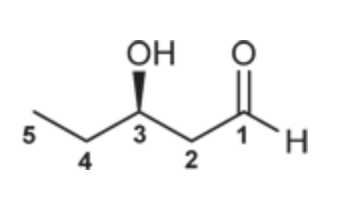
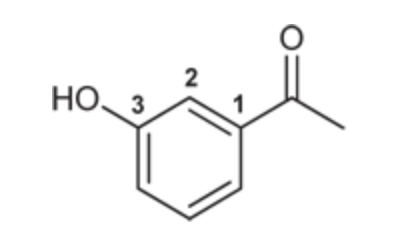
(Ch. 19) Draw the structure for each compound
meta-Hydroxyacetophenone
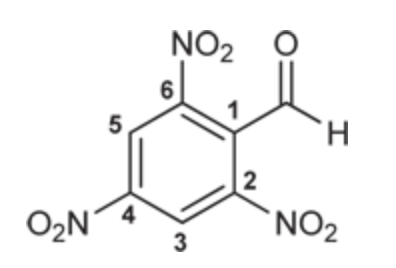
(Ch. 19) Draw the structure for each compound
2,4,6-Trinitrobenzaldehyde
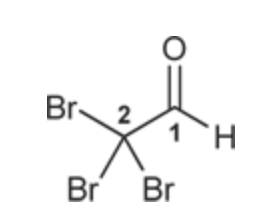
(Ch. 19) Draw the structure for each compound
Tribromoacetaldehyde
(Ch. 19) Draw the structure for each compound
(3R,4R )-3,4-Dihydroxy-2-pentanone
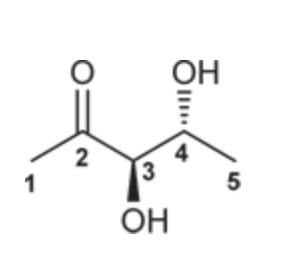
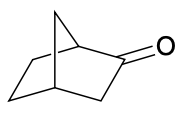
(Ch. 19) Provide a systematic (IUPAC) name for the compound below. Be careful: This compound has two chiral centers (can you find them?).
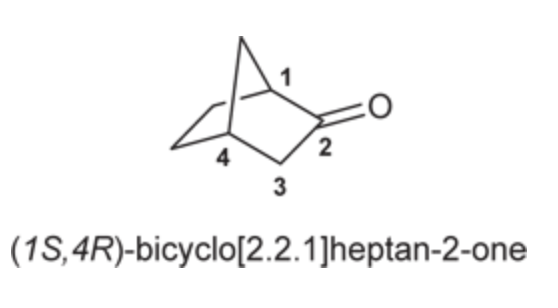
(Ch. 19) Draw all constitutionally isomeric aldehydes with the molecular
formula C4H8O and provide a systematic (IUPAC) name for each isomer.
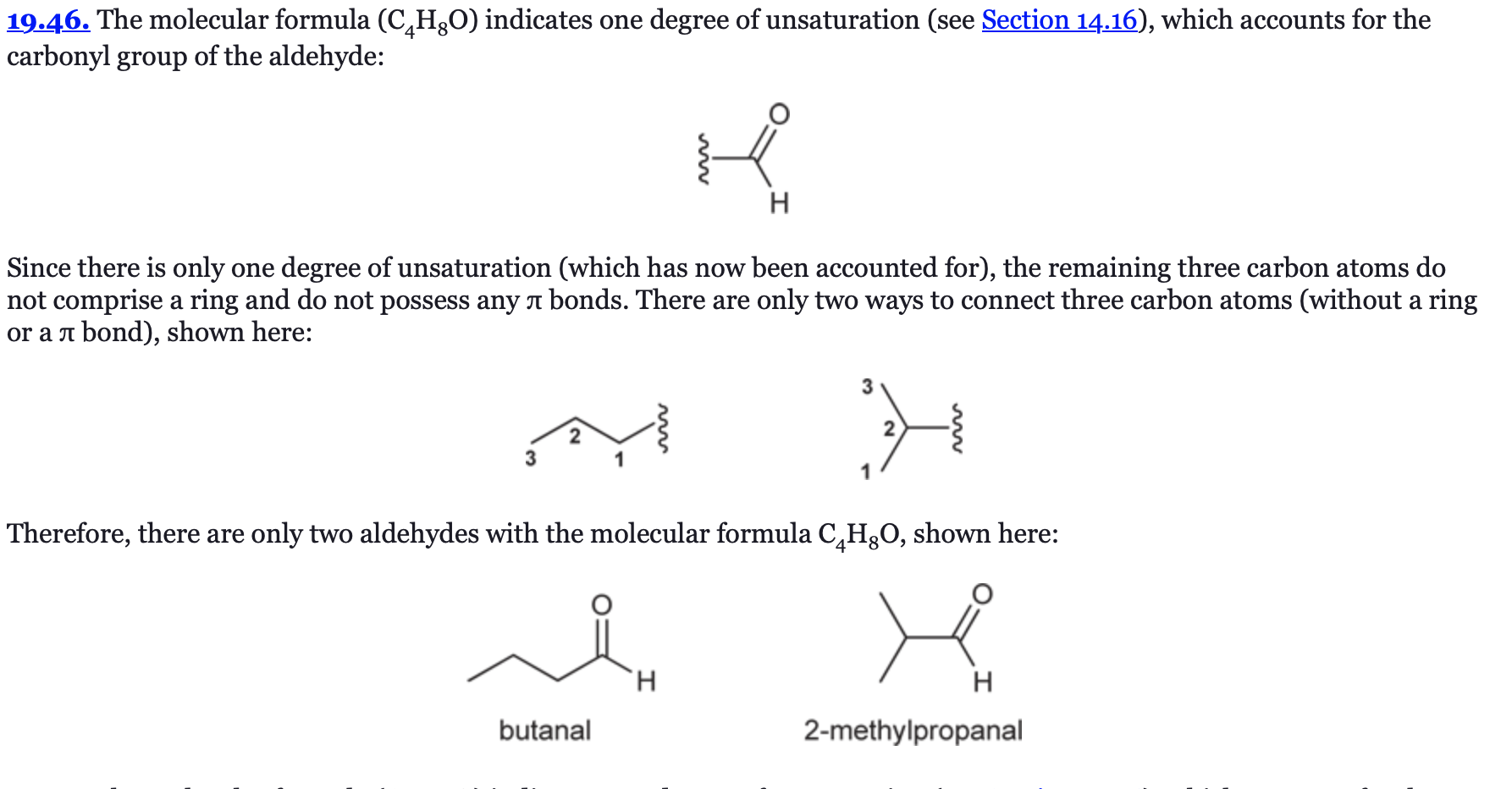
(Ch. 19) Draw all constitutionally isomeric aldehydes with the molecular formula C5H10O and provide a systematic (IUPAC) name for each isomer. Which of these isomers possesses a chiral center?
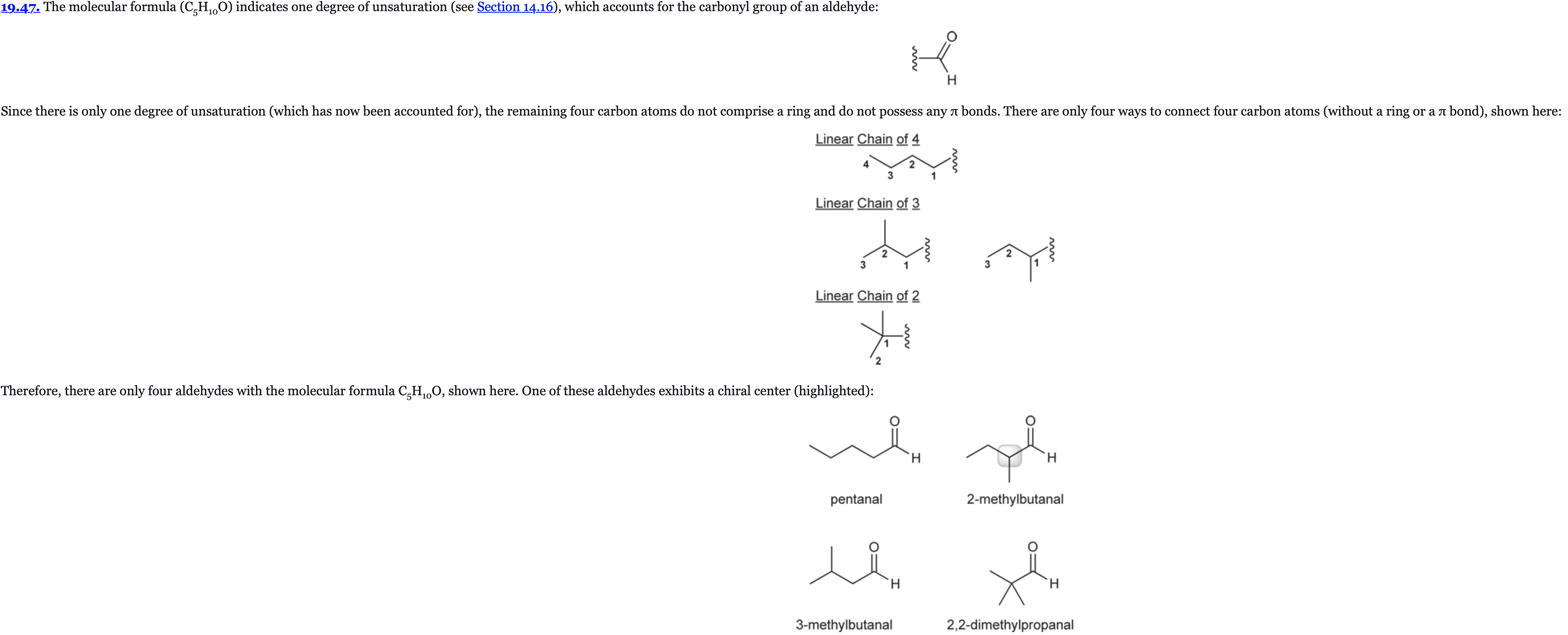
(Ch. 19) Draw all constitutionally isomeric ketones with the molecular formula C6H12O and provide a systematic (IUPAC) name for each isomer.

(Ch. 20) Provide an IUPAC and common name for the following: HO2C(CH2)3CO2H
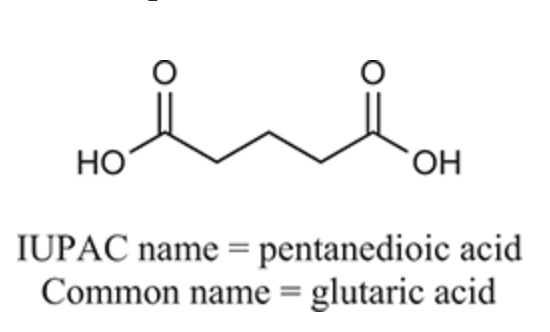
(Ch. 20) Provide an IUPAC and common name for the following: CH3(CH2)2CO2H
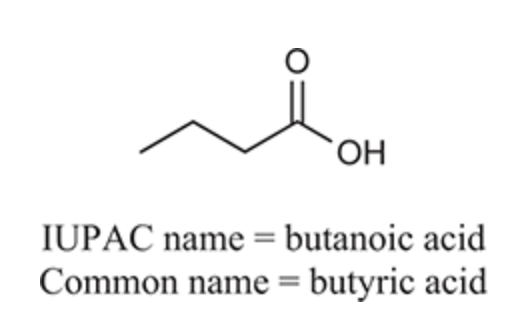
(Ch. 20) Provide an IUPAC and common name for the following: C6H5CO2H
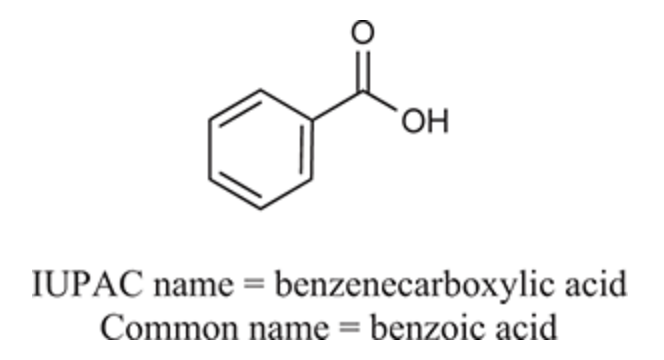
(Ch. 20) Provide an IUPAC and common name for the following: HO2C(CH2)2CO2H
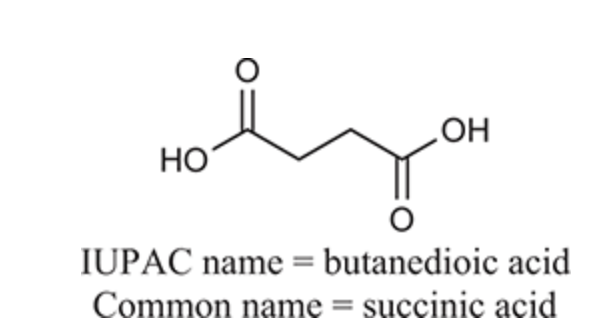
(Ch. 20) Provide an IUPAC and common name for the following: CH3COOH
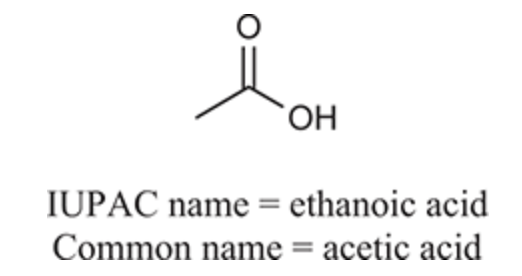
(Ch. 20) Provide an IUPAC and common name for the following: HCO2H
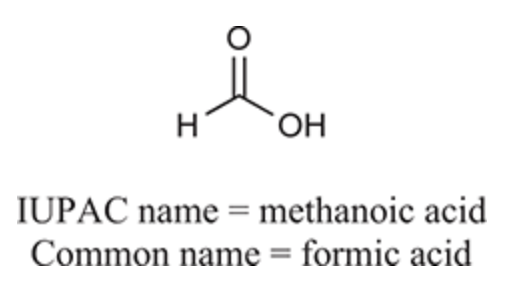
(Ch. 20) Draw the structure of the compound: Cyclobutanecarboxylic acid
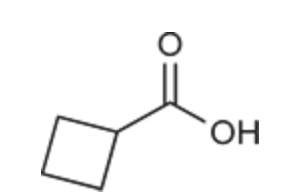
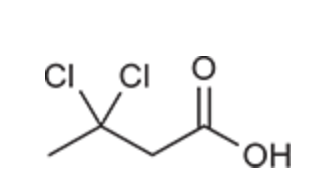
(Ch. 20) Draw the structure of the compound: 3,3-Dichlorobutyric acid
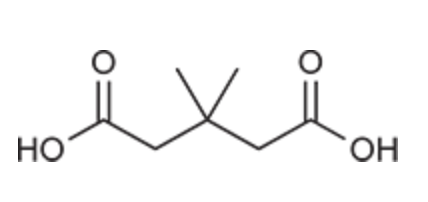
(Ch. 20) Draw the structure of the compound: 3,3-Dimethylglutaric acid
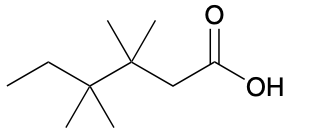
(Ch. 20) Provide an IUPAC name for the following compound:
3,3,4,4,-tetramethylhexanoic acid
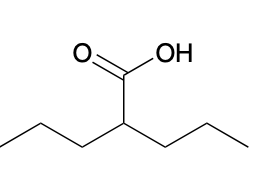
(Ch. 20) Provide an IUPAC name for the following compound:
2-propylpentanoic
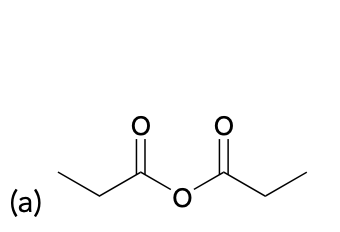
(Ch. 20) Provide a name for the following
propionic anhydride or propanoic anhydride
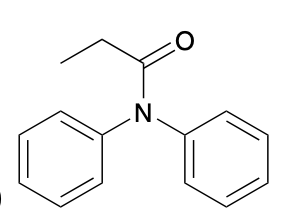
(Ch. 20) Provide a name for the following
N,N-diphenylpropionamide or N,N-diphenylpropanamide
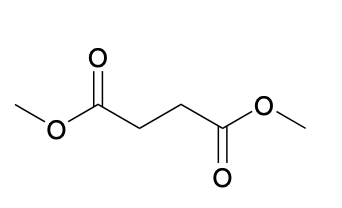
(Ch. 20) Provide a name for the following
dimethyl succinate or dimethyl butanedioate
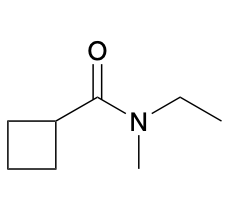
(Ch. 20) Provide a name for the following
N-ethyl-N-methylcyclobutanecarboxamide
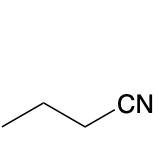
(Ch. 20) Provide a name for the following
butyronitrile or butanenitrile
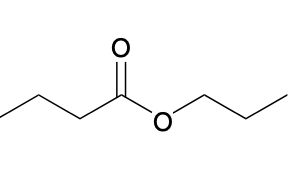
(Ch. 20) Provide a name for the following
propyl butyrate or propyl butanoate
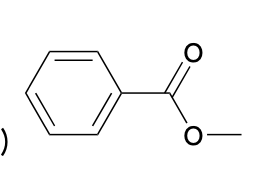
(Ch. 20) Provide a name for the following
succinic anhydride or butanedioc anhydride
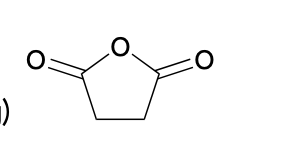
(Ch. 20) Provide a name for the following
Methyl benzoate
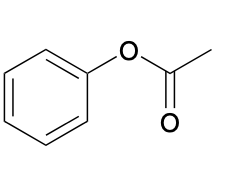
(Ch. 20) Provide a name for the following
phenyl acetate or phenyl ethanoate
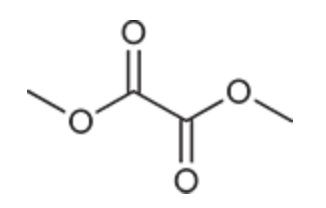
(Ch. 20) Draw a structure of the following compound: Dimethyl oxalate
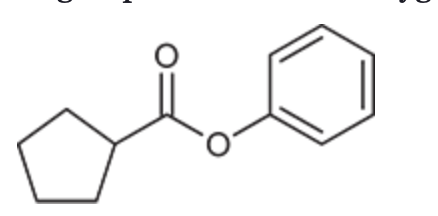
(Ch. 20) Draw a structure of the following compound: Phenyl cyclopentanecarboxylate
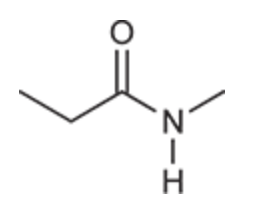
(Ch. 20) Draw a structure of the following compound: N-Methylpropionamide
(Ch. 20) Draw a structure of the following compound: Propionyl chloride
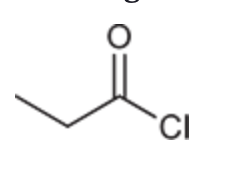
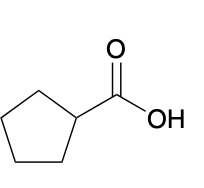
(Ch. 20) Provide a systematic IUPAC name for the following
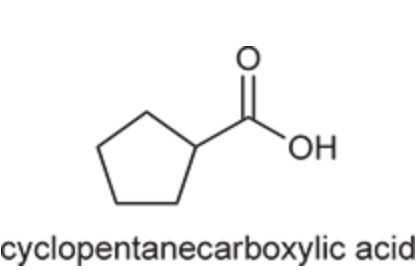
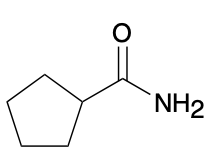
(Ch. 20) Provide a systematic IUPAC name for the following
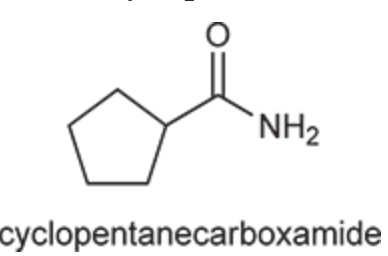
(Ch. 20) Provide a systematic IUPAC name for the following
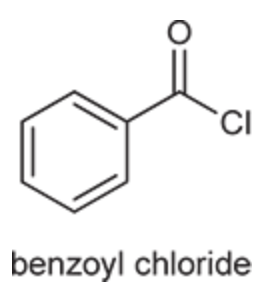
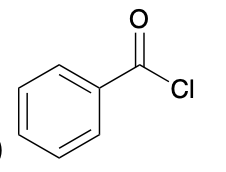
(Ch. 20) Provide a systematic IUPAC name for the following
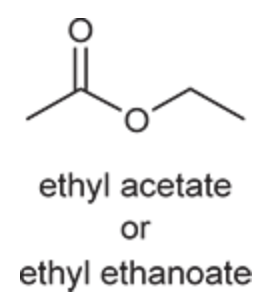
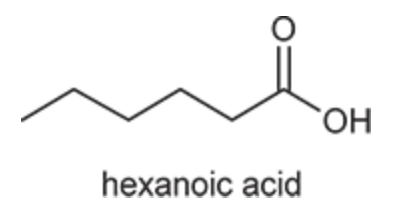
(Ch. 20) Provide a systematic IUPAC name for the following: CH3(CH2)4CO2H
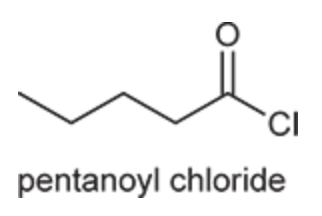
(Ch. 20) Provide a systematic IUPAC name for the following: CH3(CH2)3COCl
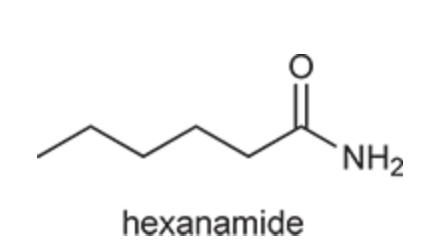
(Ch. 20) Provide a systematic IUPAC name for the following: CH3(CH2)4CONH2
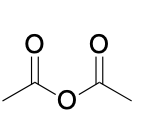
(Ch. 20) Identify the common name for the following
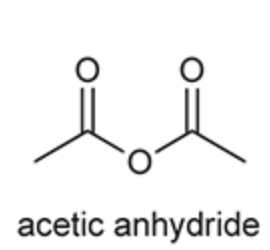
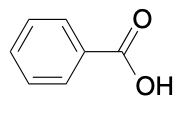
(Ch. 20) Identify the common name for the following
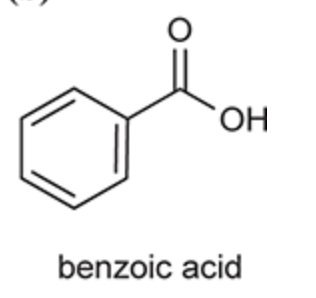
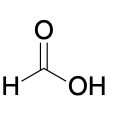
(Ch. 20) Identify the common name for the following
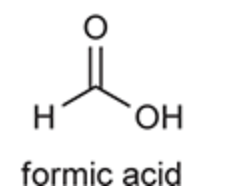
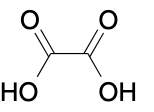
(Ch. 20) Identify the common name for the following
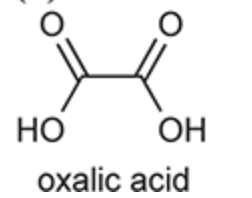
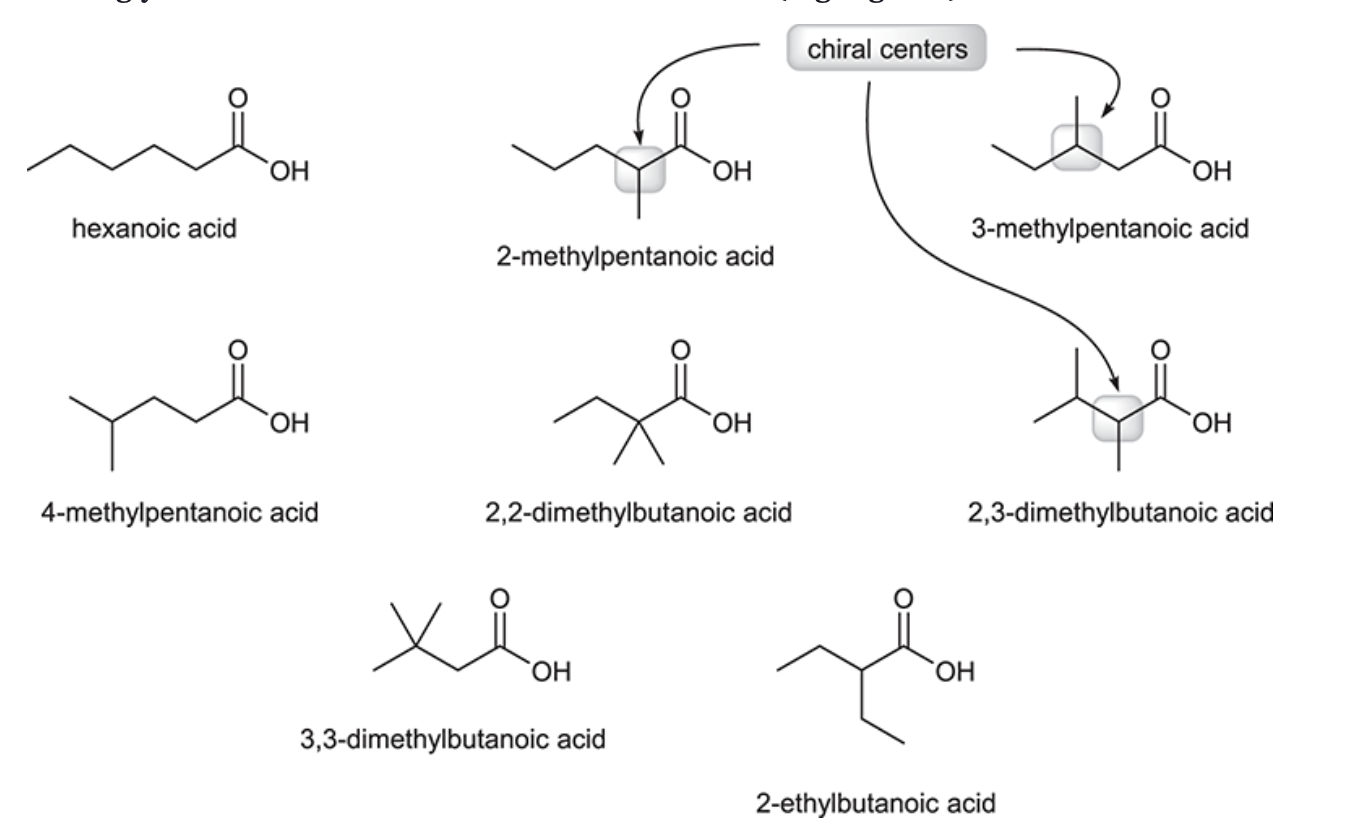
(Ch. 20) Draw the structures of eight different carboxylic acids with the molecular formula C6H12O2. Then, provide a systematic name for each compound and identify which three isomers exhibit chiral centers.
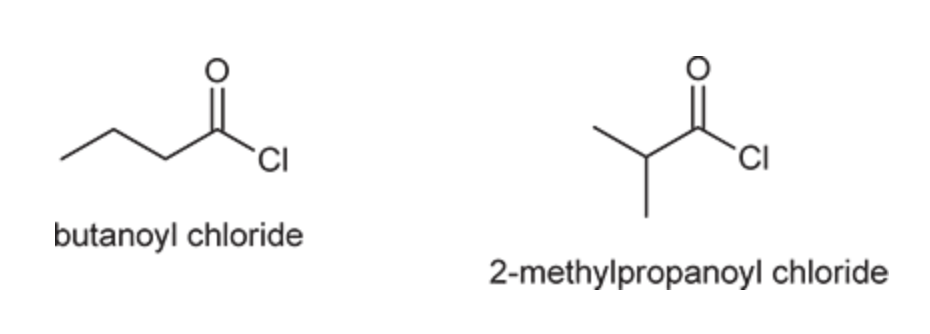
(Ch. 20) Draw and name all constitutionally isomeric acid chlorides with the molecular formula C4H7ClO. Then provide a systematic name for each isomer.
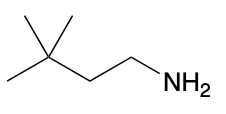
(Ch. 22) Assign a name for the following compound
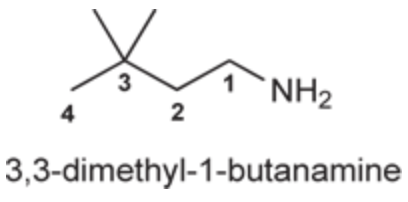
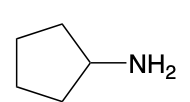
(Ch. 22) Assign a name for the following compound
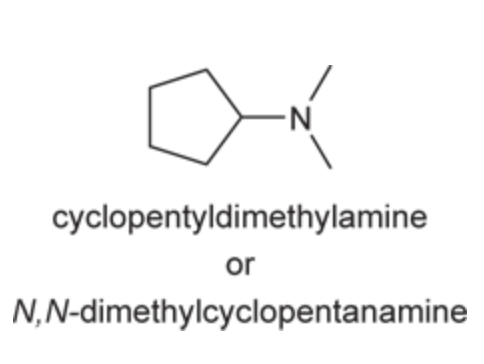
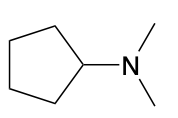
(Ch. 22) Assign a name for the following compound
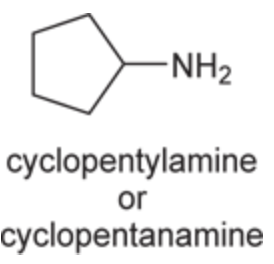
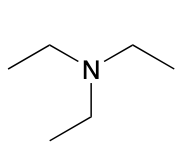
(Ch. 22) Assign a name for the following compound
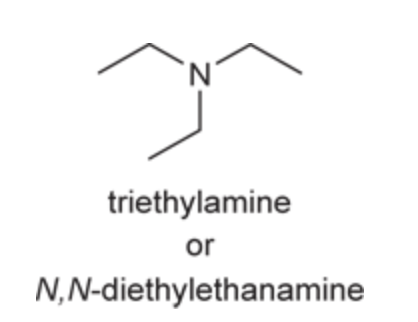
(Ch. 22) Assign a name for the following compound
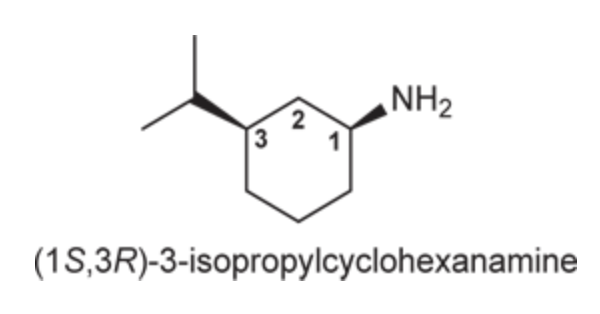
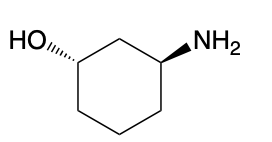
(Ch. 22) Assign a name for the following compound