4- Stereochemistry
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
molecules with the same chemical formula but different structures
isomers
molecules that have the same chemical formula but different connectivity/location of atoms
constitutional isomers (structural isomers)
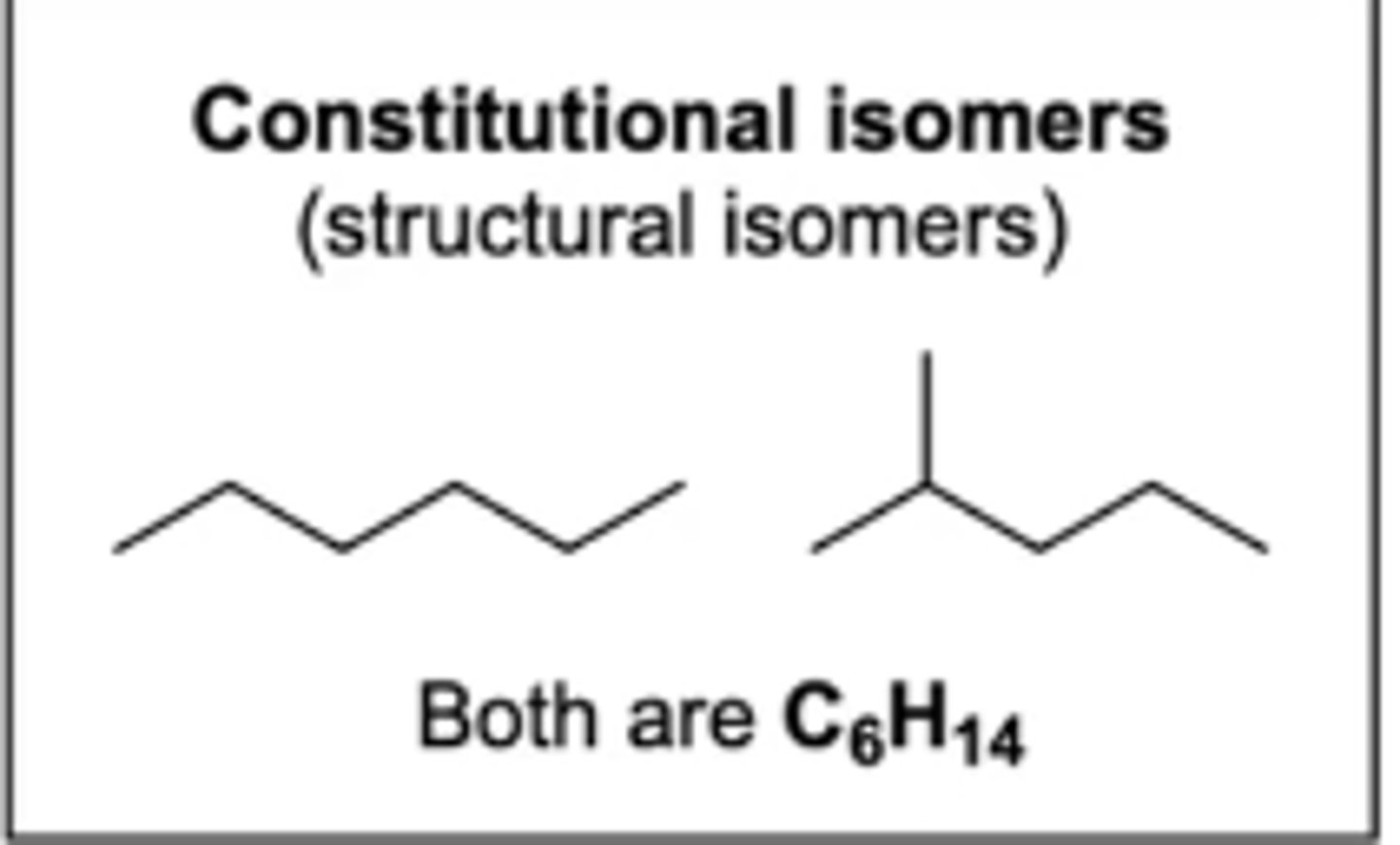
molecules that have the same chemical formula, same connectivity/location of atoms, and are superimposable
they're the same molecule
molecules that have the same chemical formula, same connectivity/location of atoms, are mirror images of each other, but are not superimposable
enantiomers (called stereoisomers)
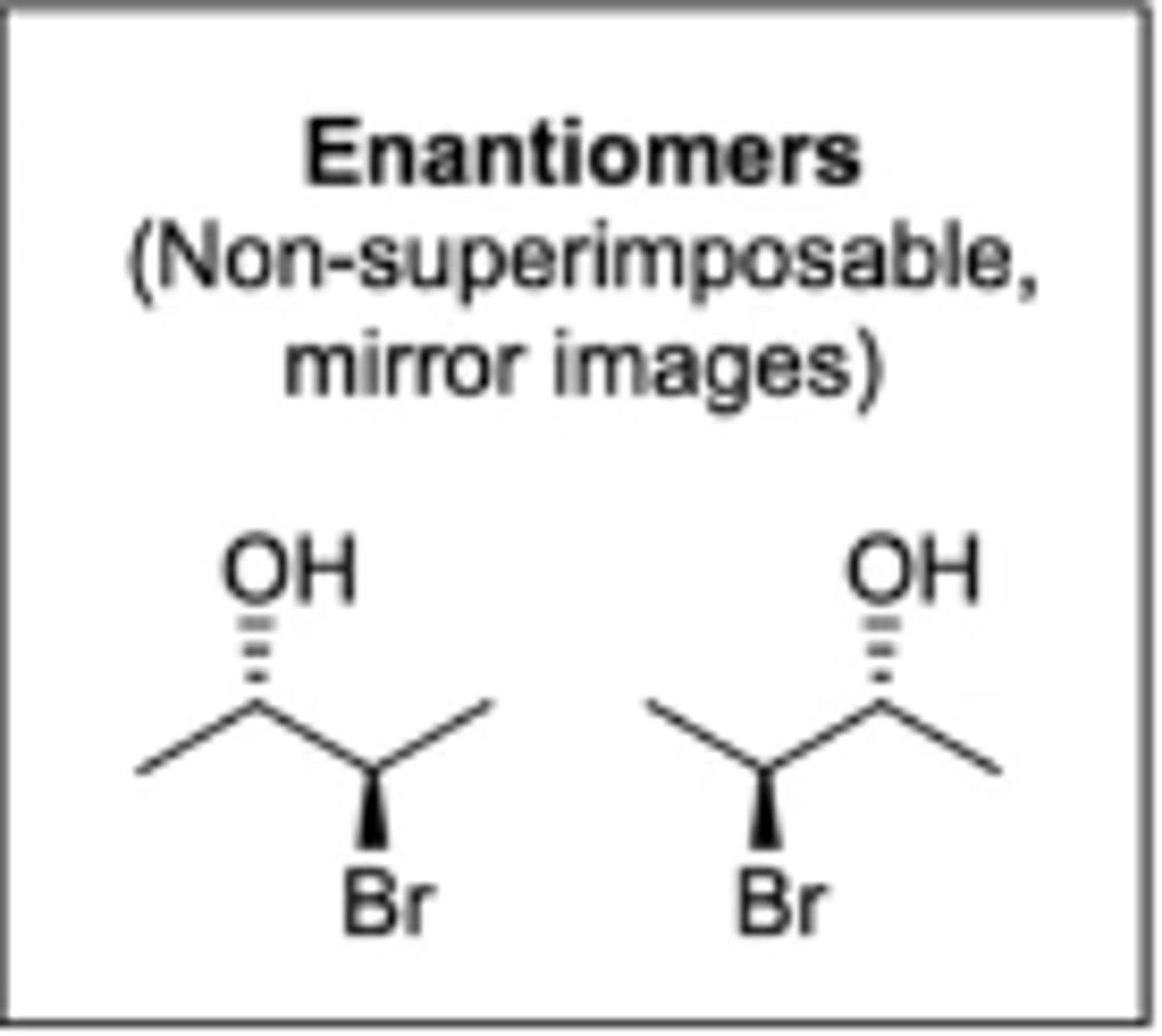
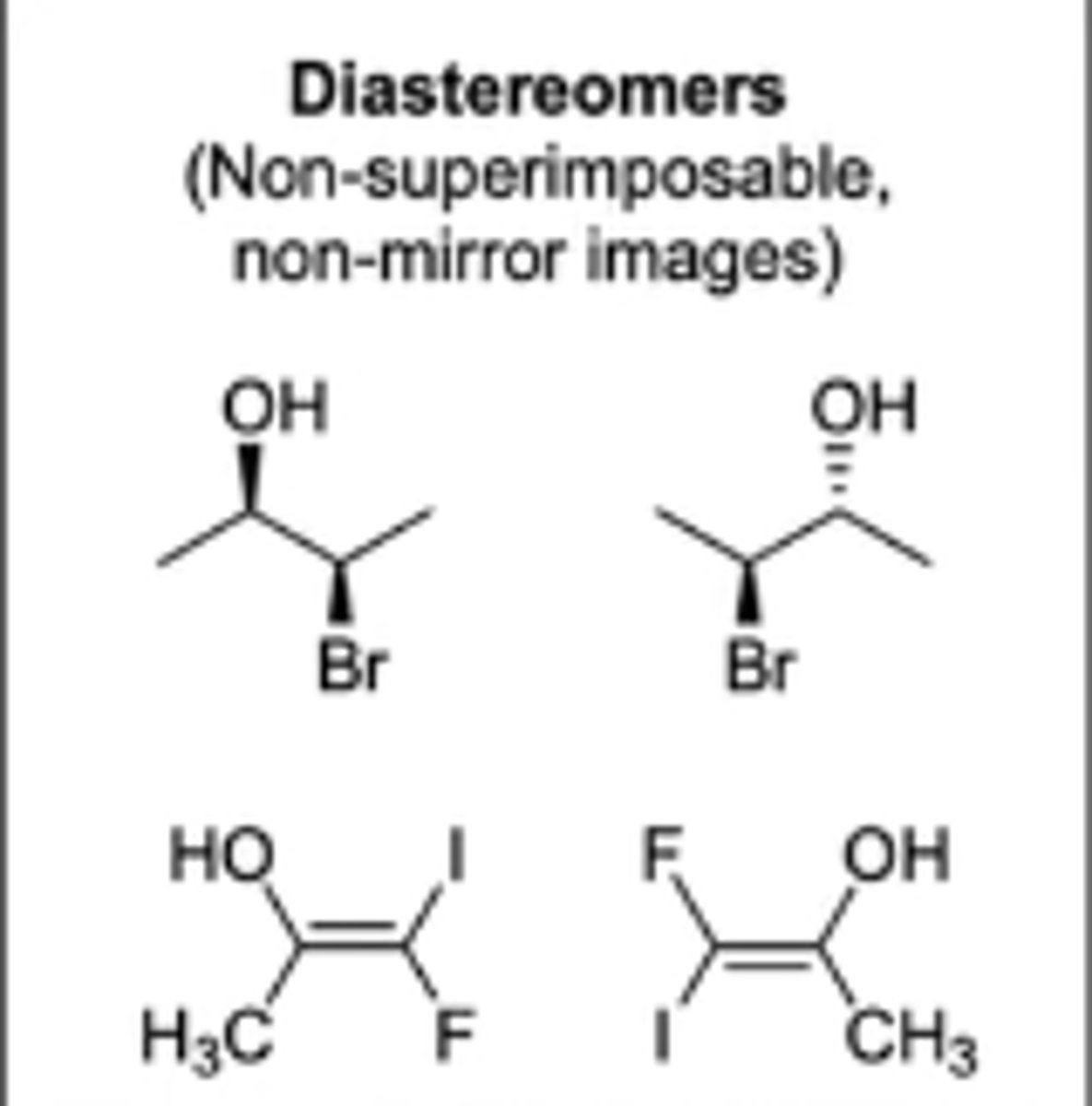
molecules that have the same chemical formula, same connectivity/location of atoms, are not superimposable, and are not mirror images of each other
diastereomers (called stereoisomers)
what are stereocenters/chiral centers?
tetrahedral center molecules with four different groups around it
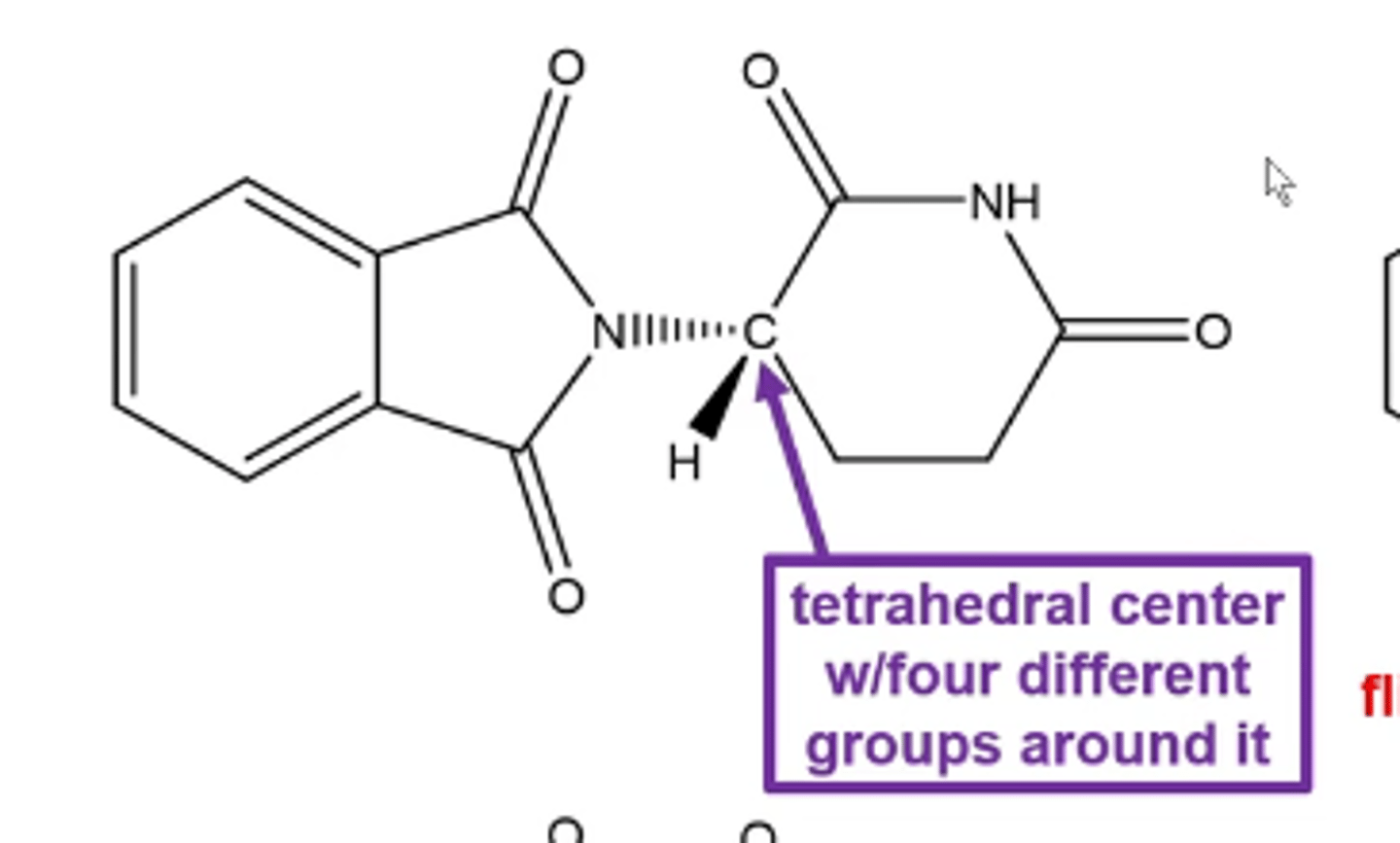
non-superimposable mirror-image molecules
-almost always involves having a carbon atom bonded to four different groups in a tetrahedral geometry
enantiomers
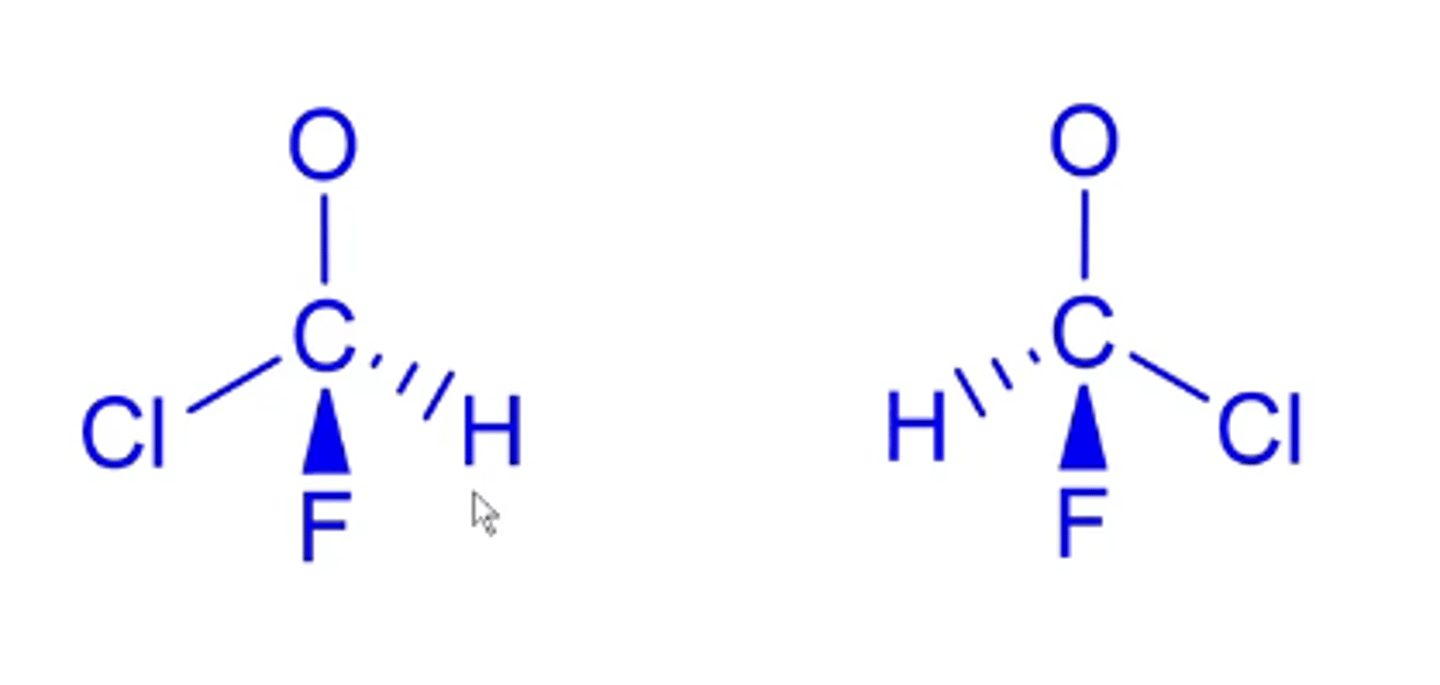
how do you use the R/S naming system for enantiomers?
1. find stereocenter atom
2. prioritize the four appendages coming off of the stereocenter atom (higher priority=highest atomic number; ties go out to the next atom beyond that)
3. number substituents from highest priority to lowest priority
4. if lowest priority substituent is a wedge, make it a dash and subsequently change the direction of the circle
Clockwise=R
Counterclockwise=S
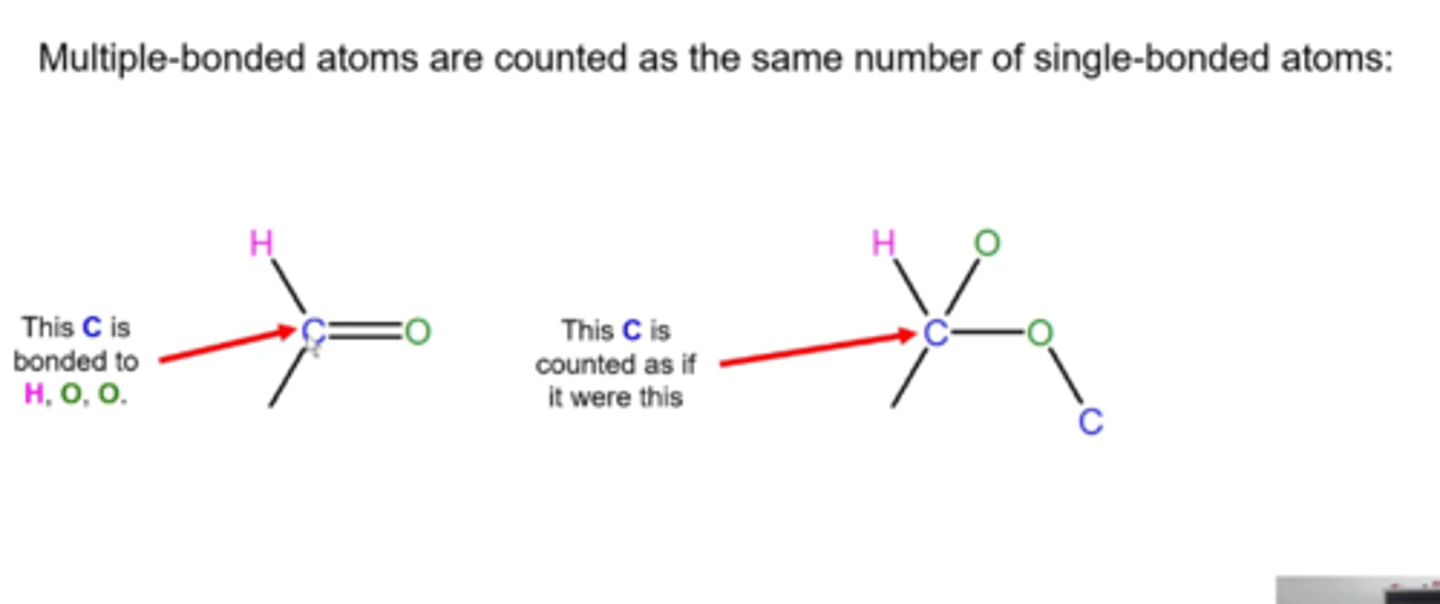
what are the three types of diastereomers?
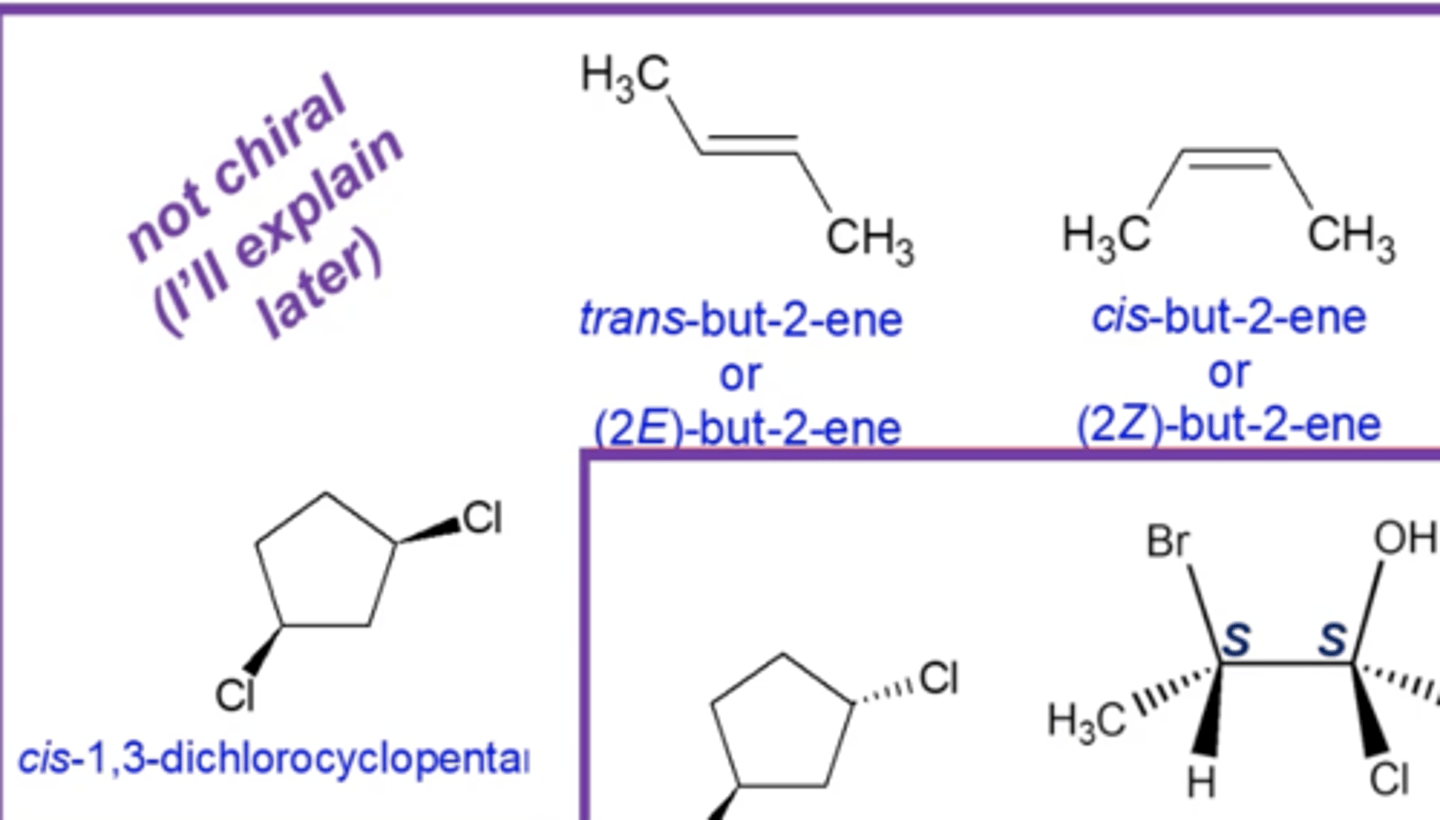
-cis/trans isomers of ringed compounds
-cis/trans or E/Z isomers of alkenes
-stereoisomers with multiple stereocenters that do NOT have exactly opposite R,S configurations
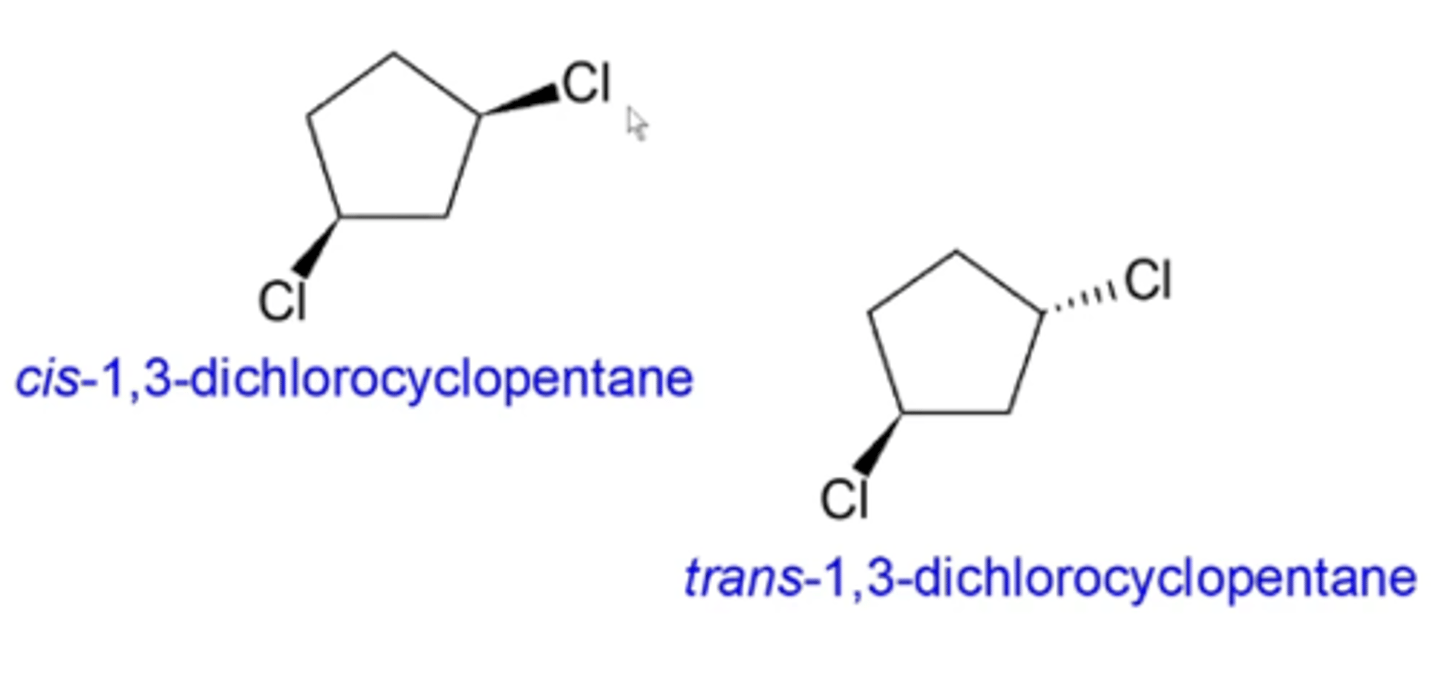
cis/trans isomers of ringed compounds:
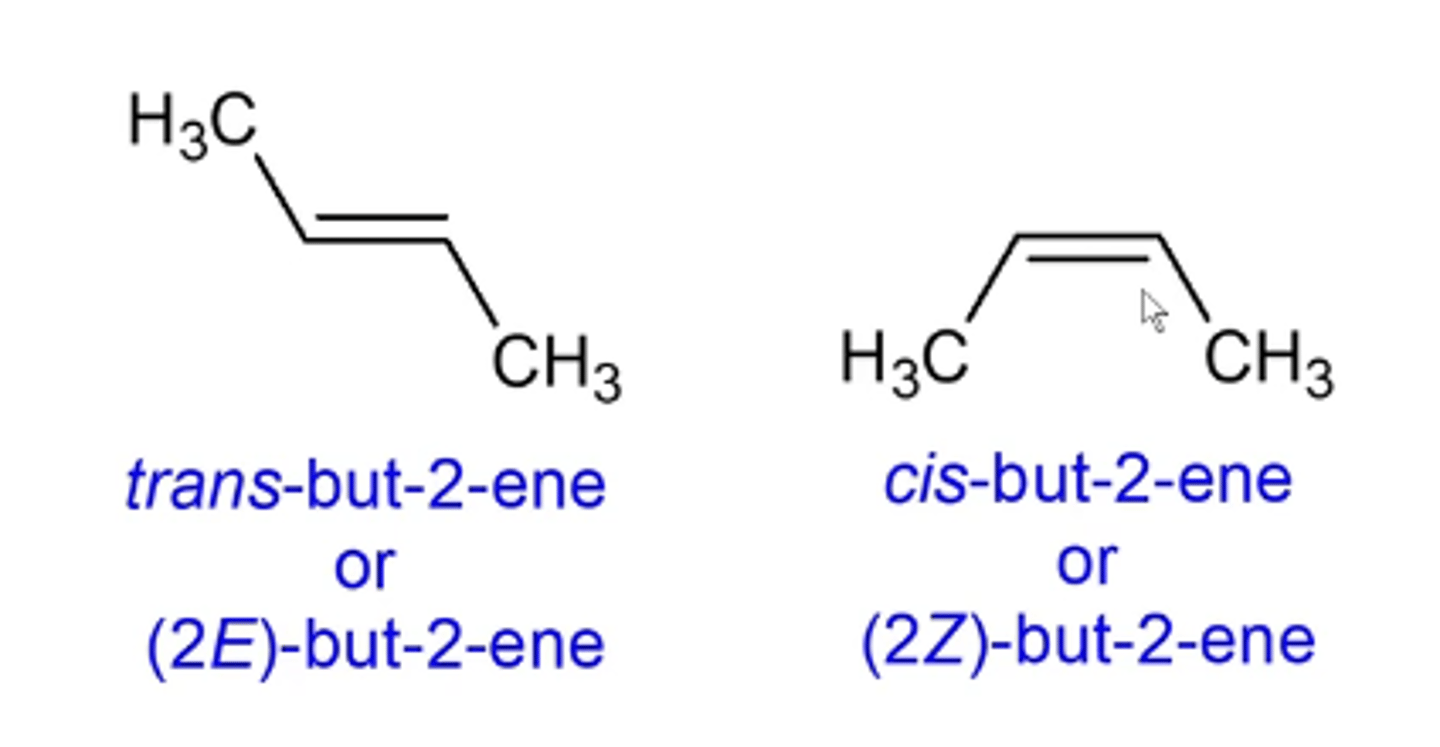
cis/trans of E/Z isomers of alkenes
stereoisomers with multiple stereocenters that do NOT have exactly opposite R,S configurations:
if stereoisomers have exactly opposite R,S configurations, then they are enantiomers. If they have the same, then they are the same molecule. If they're somewhere in between, they're diastereomers
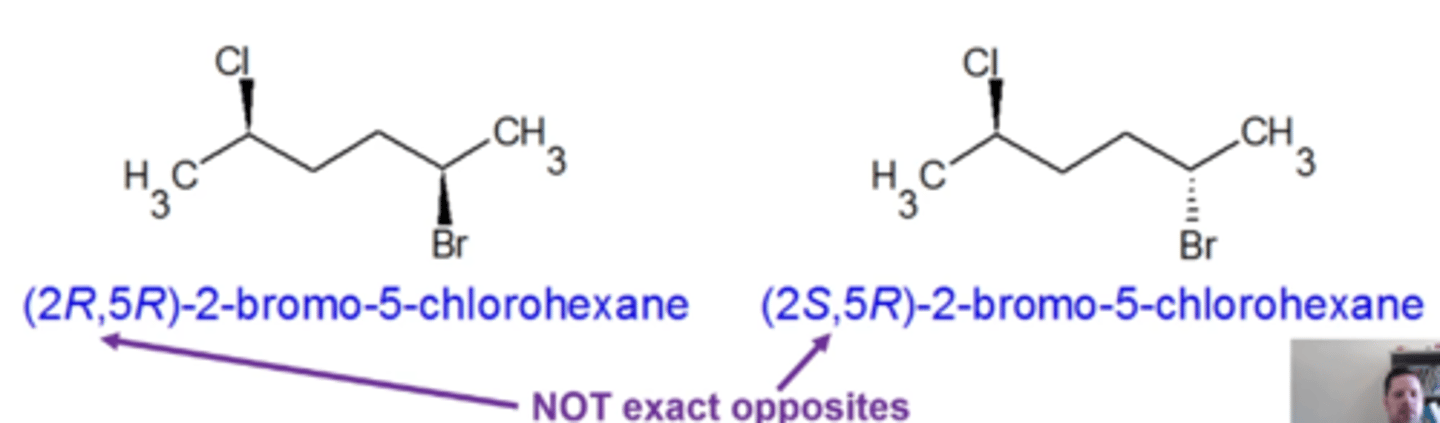
how do you determine how many possible stereoisomers a molecule can have?
# of possible stereoisomers = 2^n
(n= # of stereocenters)
which types of diastereomers are optically active? (chiral)
trans isomers of ringed compounds and stereoisomers with multiple stereocenters that do NOT have exactly opposite R,S configurations
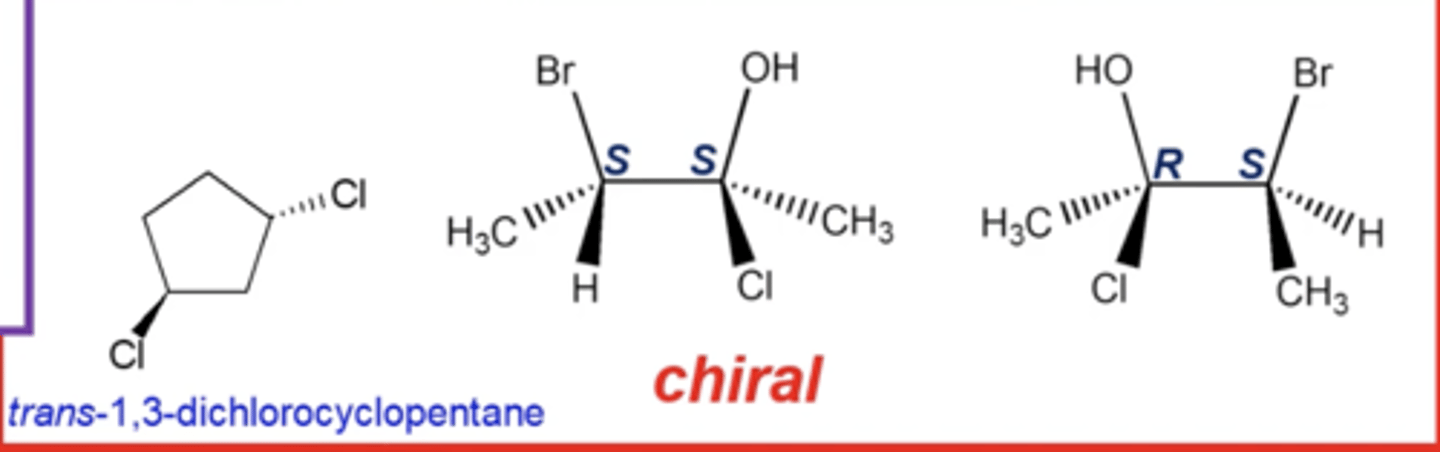
which types of diastereomers are optically inactive (achiral)?
cis isomers of ringed compounds and cis/trans or E/Z isomers of alkenes
what is the machine that fires plane polarized light down a tube to test whether a solution of molecules will rotate it?
polarimeter
what are chiral molecules that rotate plane-polarized light clockwise called?
dextarotatory, D
enantiomers rotate light by the exact same amount as each other, but in opposite directions
what are chiral molecules that rotate plane-polarized light counterclockwise called?
levorotatory, L
enantiomers rotate light by the exact same amount as each other, but in opposite directions
why is a 50/50 mixture of two enantiomers (a racemic mixture) achiral/optically inactive?
opposite enantiomers counteract the effects of their siblings, producing a net zero effect on the light
why are meso compounds achiral?
meso compounds: a compound that has 2 or more stereocenters and can be bisected by a line a symmetry (is a mirror image)
-meso compounds don't have enantiomers because their enantiomers would be the same
how do physical properties of corresponding enantiomers compare?
they have the same physical properties as each other: the same boiling points, solubilities, melting points, and so forth. Thus, it is very hard to separate enantiomers from each other once they're mixed together
what are the only major physical differences between enantiomers?
their direction in which they rotate plane-polarized light AND their effect on biological systems because both plane polarized light and living systems are chiral
how do the physical properties of corresponding diastereomers compare?
they DO have different physical properties (boiling point, solubility, melting point, etc), so they can be more easily separated from each other
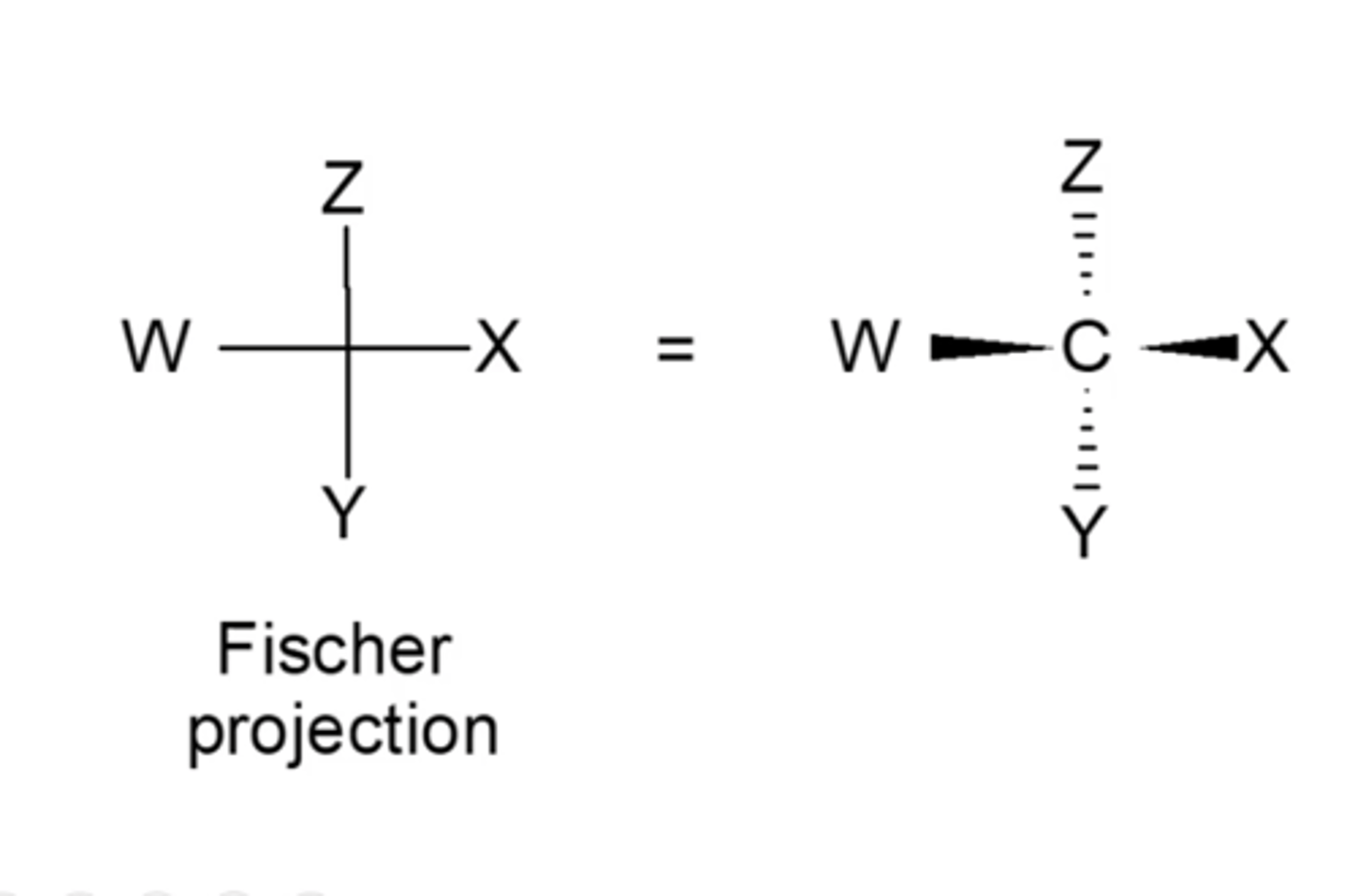
what are the general rules for Fischer projections?
-horizontal bonds go towards you
-vertical bonds go away from you
*typically, you want the all-carbon spine to go vertically
how do you categorize sugars as "D" or "L"?
when you draw a Fischer projection of a sugar, if the bottommost OH (green) along the spine of the sugar points to the right, it's a D sugar. If it points to the left, then it's an L sugar
(this is completely unrelated to R/S)
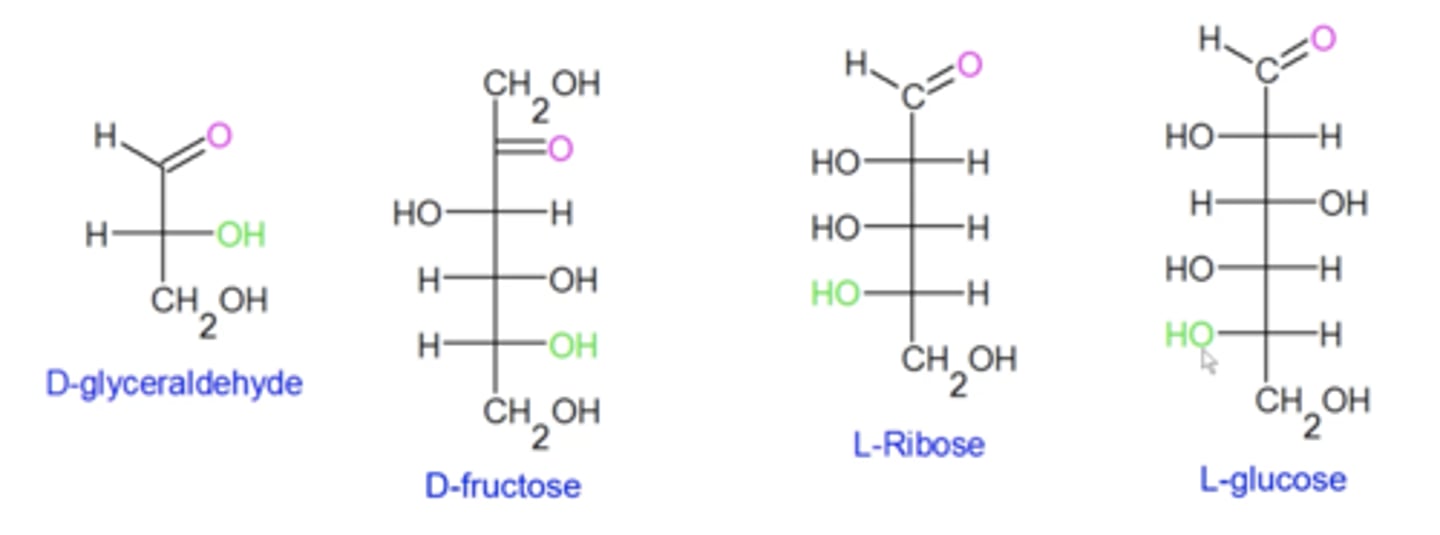
are all naturally-occurring amino acids L or D?
L