An atom is the smallest part of an element that can exist. Chemical reactions always involve the formation of one or more new substances, and often involve a detectable energy change. Compounds contain two or more elements chemically combined in fixed proportions and can only be separated into elements by chemical reactions. A mixture consists of two or more elements or compounds not chemically combined together. The chemical properties of each substance in the mixture are unchanged. Mixtures can be separated by physical processes such as filtration, crystallisation, simple distillation, fractional distillation and chromatography. These physical processes do not involve chemical reactions and no new substances are made.
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
Compounds
contain two or more elements chemically combined in a fixed ratio.
How to separate compounds
By chemical reactions
Mixture
consists of two or more elements or compounds not chemically combined together.
Filtration
Filtration is a method to separate a solid and a liquid. The solid is collected on a filter paper, while the liquid passes through it and is collected in a separate container.
Crystallization
Crystallisation is a method used to remove impurities from solids. In this method, the solid to be purified is dissolved in water and then filtered to remove impurities. The solution is heated such that water evaporates to get a saturated solution. Later, as it cools, the pure solid crystallises out in pure form
Simple distillation
used to separate two liquids with a large difference in boiling points, such as ethanol. and water. The mixture must be carefully heated and the temperature controlled, so that only the liquid with the lower boiling point evaporates. This can then be collected using a condenser
Fractional distillation
mixture of several substances, such as crude oil, is distilled and the evaporated components are collected as they condense at different temperatures. is used to separate the different liquids from a mixture of liquids.
Size and mass of atoms
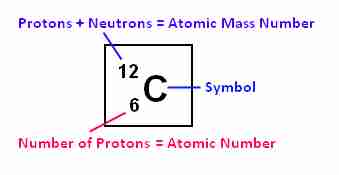
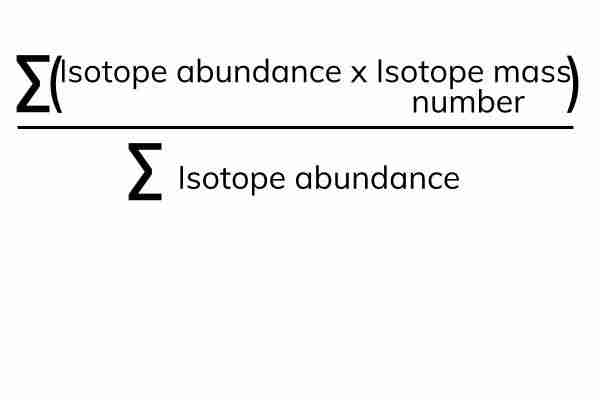
Relative atomic mass
Elements in the same group…
have similar to chemical properties because they have the same number of electrons in the outer shell
reactivity in metals, no metals, and periods
metals: reactivity increases down the group.
No metals: reactivity increases up the group
Reactivity increases left to right in a period
Physical properties of metals
Shiny, malleable, sonorous, good conductors of heat and electricity
Chemical properties of metals
ability to form positive ions, react with acid to produce hydrogen gas, and to corrode
Physical properties of non metals
dull, brittle, poor conductors of heat and electricity low density
Chemical properties of non metals
ability to form negative ions, react with metals to form ionic compounds, form covalent bonds with other non metals
How do properties of the elements in group 0 depend on the outer shell of electrons of the atom?
They have a full outer shell so they don't need to lose, gain, or share electrons
predict properties of given trends down group 0
The reactivity decreases down the group as the atom gets larger.
• density increases so melting/boiling point also increases down the group
How do properties of the elements in Group 1 depend on the outer shell of electrons
Reactivity increases going down the group because the atom gets larger. The force of attraction between the electron and nucleus is weaker so the electron is more easily lost.
predict properties of given trends down group 1
Reactivity increases down the group because the atom gets larger. Melting and boiling point decreases.
Describe the reactions of the alkali metals with water
Forms a metal hydroxide and hydrogen gas
Li -