T1 atomic structure and the periodic table
1/30
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
relative mass of an electron
1/2000
isotope definition
atoms of an element with the same number of protons but diff num of neutrons
relative atomic mass definition
the weighted mean mass of an atom of an element, compared to 1/12th of the mass of an atom of C-12
relative isotopic mass
it is the mass of an atom of an isotope compared to 1/12th of the mass of a carbon-12 atom
relative atomic mass formula
(abundance A x Mr(A)) + (abundance B x Mr (B))
total abundance
when should term 'relative formula mass' be used instead of 'relative molecular mass'
used for compounds with giant structures
state how relative abundance of two isotopes can be found
compare intensity of signal/ number of particles of each isotope detected
in mass spectrometer
predicting mass spectra for diatomic molecules
make abundances decimals out of 1
identify the abundances of all possible isotope combinations using a table and multiplying them. all isotopes both across and down to form multiplication table
any molecules that are the same, add the abundances together
times all abundances by 100 to find abundance as a percentage, or divide all abundances by the smallest abundance to find a whole number ratio
plot results using Mr as x axis and abundance as y axis
what is the M+1 peak?
aka molecular ion peak
it is the last peak on the spectra
it shows the Mr unfragmented molecule
(+1 only refers to charge)
explain how atomic emission spectra provides evidence for ideas on electronic configurations
atomic emission spectra provide evidence for the existence of quantum shells
clear lines/ frequencies for different energy levels
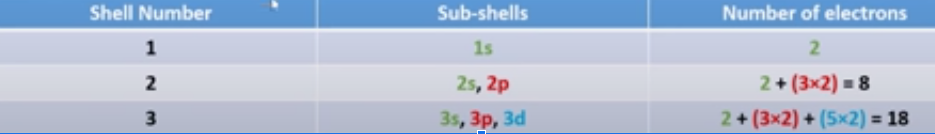
sub-shell
s: 1 orbital = can hold 2 electrons
p: 3 orbitals = 6
d: 5 orbitals = 10
f: 7 orbitals = 14
only until 4p⁶ needed
orbital
region within an atom that can hold up to 2 electrons with opposite spins
subshell orbital shapes
s: 1 orbital, spherical
p: 3 orbitals, dumbell shape, perpendicular to each other, x,y,z axes
spin pairing
when 2 electrons occupy 1 orbital they spin in opposite directions
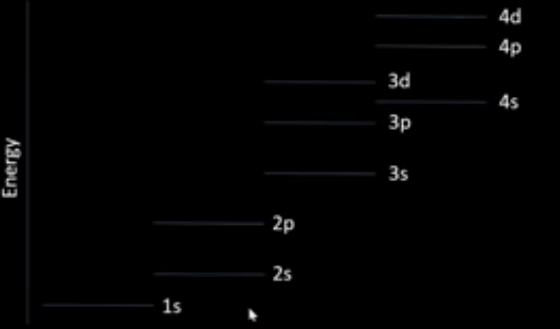
energy levels of subshells
we fill from lowest to highest
e⁻s fill subshells singly before pairing up due to e⁻ repulsion
which cells fill up first in a transition metal?
4s
except Cr and Cu
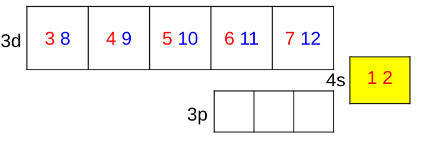
Cr and Cu electronic configuration
4s subshell donates e⁻ to form full/singly filled 3d subshell
Priority: to have a perfect 3d subshell: more stable
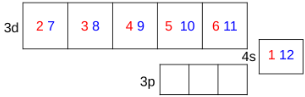
In which order do transition metals lose electrons to form ions?
always 4s first, then 3d
first ionisation energy
energy required to remove 1 electron from each atom in 1 mole of gaseous atoms to form one mole of gaseous 1+ ions.
(energy is req to overcome electrostatic attr between nucleus and e⁻)
successive ionisation energies
energy required to remove 1 electron from each ion in 1 mole of gaseous (1+) ions to form one mole of gaseous (2+) ions
(second ionisation energy)
explain trend in values of 1st ionisation energies down group
first ionisation energy decreases down group
bc although num of protons is increasing
outer e⁻ is one shell of e⁻s further from the nucleus
larger atomic radius and more shells of electrons --> more shielding
this outweighs/ exerts a greater influence than greater positive/nuclear charge
provides evidence for e⁻ shells
explain why the 1st ionisation energy of generally increases across period
bc atomic number incrs by one
and e⁻ removed is from same (sub)shell and has similar shielding
sub necessary if increasing
explain how different factors influence ionisation energies
number of protons
greater nuclear charge = stronger force of attraction from nucleus to (outer) e⁻electron shielding
outer e⁻ in same quantuum shell = similair levels of shielding
outer e⁻ in higher energy level = larger atomic radius and more shells of electrons --> more shielding, drop in force of attraction between nucleus and (outer) e⁻
this outweighs/ exerts a greater influence than greater positive/nuclear charge
electron subshell from which electron is being removed
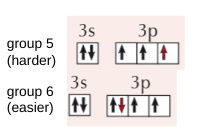
explain drop between group 6 after group 5 in 1st ionisation energies
fully singly filled/ fully filled subshells are more stable than partially filled ones
so have higher ionisation energies
in group 6 spin pairing has occured
resulting in an increase in repulsion between electrons
so electron lost more easily
explain drop between for group 3 after group 2 in 1st ionisation energies
electron removed from 3 is from new subshell, p instead of s
extra shielding and distance from nucleus
this outweighs increase in nuclear charge
--> evidence for electron subshells
explain big jump in successive ionisation energies
"new shell broken into":
e⁻ lost from shell closer to nucleus
previous from same shell w similar shielding
why do successive ionisation energies increase
same number of protons attracting a decreasing number of electrons
decreasing repulsion amongst remaining electrons
what determines the chemical properties of an element
electronic configuration
same number of electrons in outer shell
electronic configuration / n of e⁻ in outer shell govern their chemical reactions
explain periodicity in terms of a repeating pattern across different periods
a trend/ pattern of repeating physical and chemical properties with increasing atomic number
explain pattern in atomic radii across a period
atomic radii decrease across period
proton number and nuclear charge increases
attraction increases
extra electrons gained across period added to outer energy level so do not provide extra shielding
explain reason for trend in melting/ boiling temps for elements in a period
at start of period bonding is metallic
metallic bonding gets stronger as number of delocalised electrons in a metal increases/ charge on cation increases
middle of period (B and C) have giant covalent structure
a lot of energy is required to break covalent bonds
at the end of the period elements form simple molecules
with weak london forces between them
noble gases are monoatomic resulting in very weak london forces