Electron Configuration of Atoms + Periodic Relationship Among Elements
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
Pauli Exclusion Principle
No two electrons in an atom can have the same set of quantum numbers.
Hund’s Rule
The most stable arrangement of electrons in subshells is the one with the greatest number of parallel spins.
AUFBAU Principle
As protons are added one by one to the nucleus to build up each element, electrons are similarly added to the atomic orbitals.
Diamagnetic
Magnetic Properties of Atoms:
All electrons are paired.
Paramagnetic
Magnetic Properties of Atoms:
There is at least one unpaired electron.
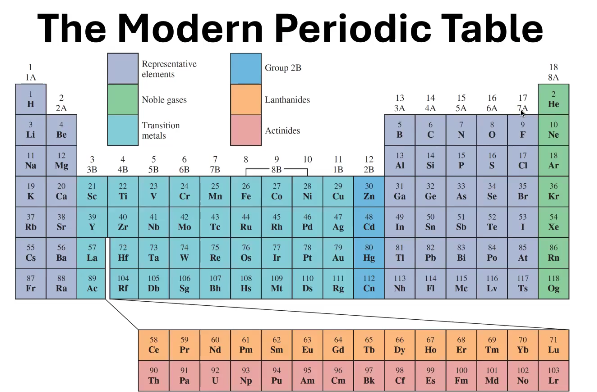
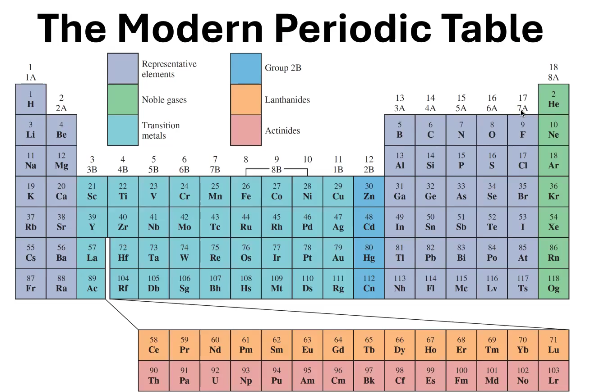
1A - 7A
In what groups can you find the Representative Elements?
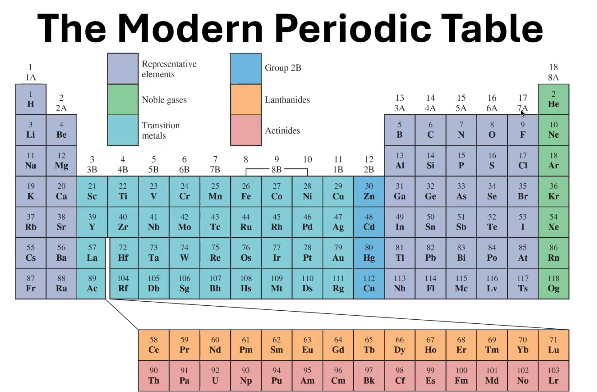
8A
In what groups can you find the Noble Gases?
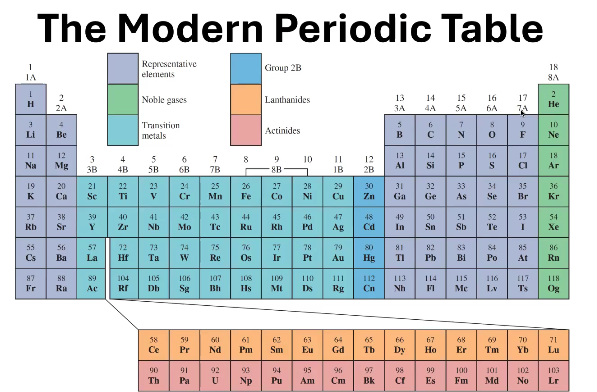
1B - 8B (except 2B)
In what groups can you find the Transition Metals?
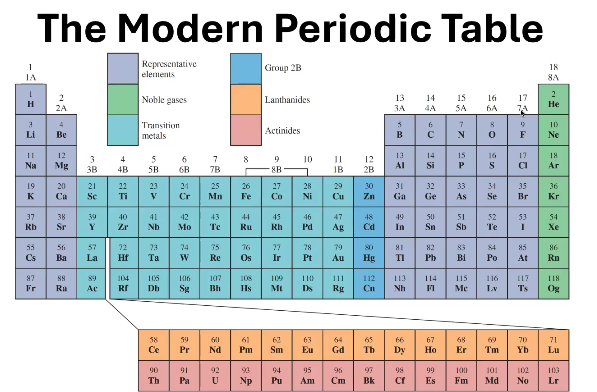
Where can you find 2B?
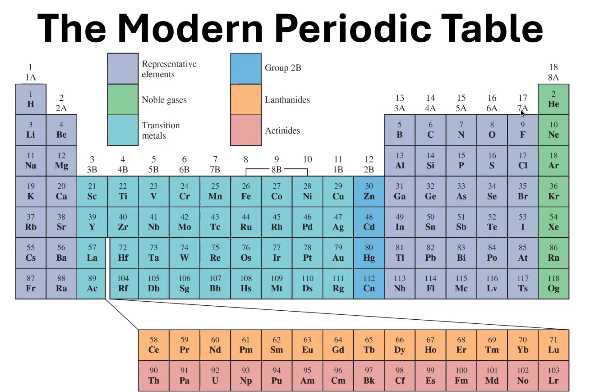
6
In what period can you find the Lanthanides?
7
In what period can you find the Actinides?
Core Electrons
Electrons with completely filled subshells.
Valence Electrons
Electrons located in the outermost shell of an atom (incomplete).
Cations
Positively Charged Electrons
Anions
Negatively Charged Electrons
Isoelectronic Species
Atoms, ions, or molecules that have the same number of electrons and the same electron configuration.
F-,
O2-
N3-
Na+
Al3+
What elements are the Isoelectronic Species?