Group 0: Groups in the periodic table: Chemistry: GCSE (9:1)
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
Name of group 0
Noble gases
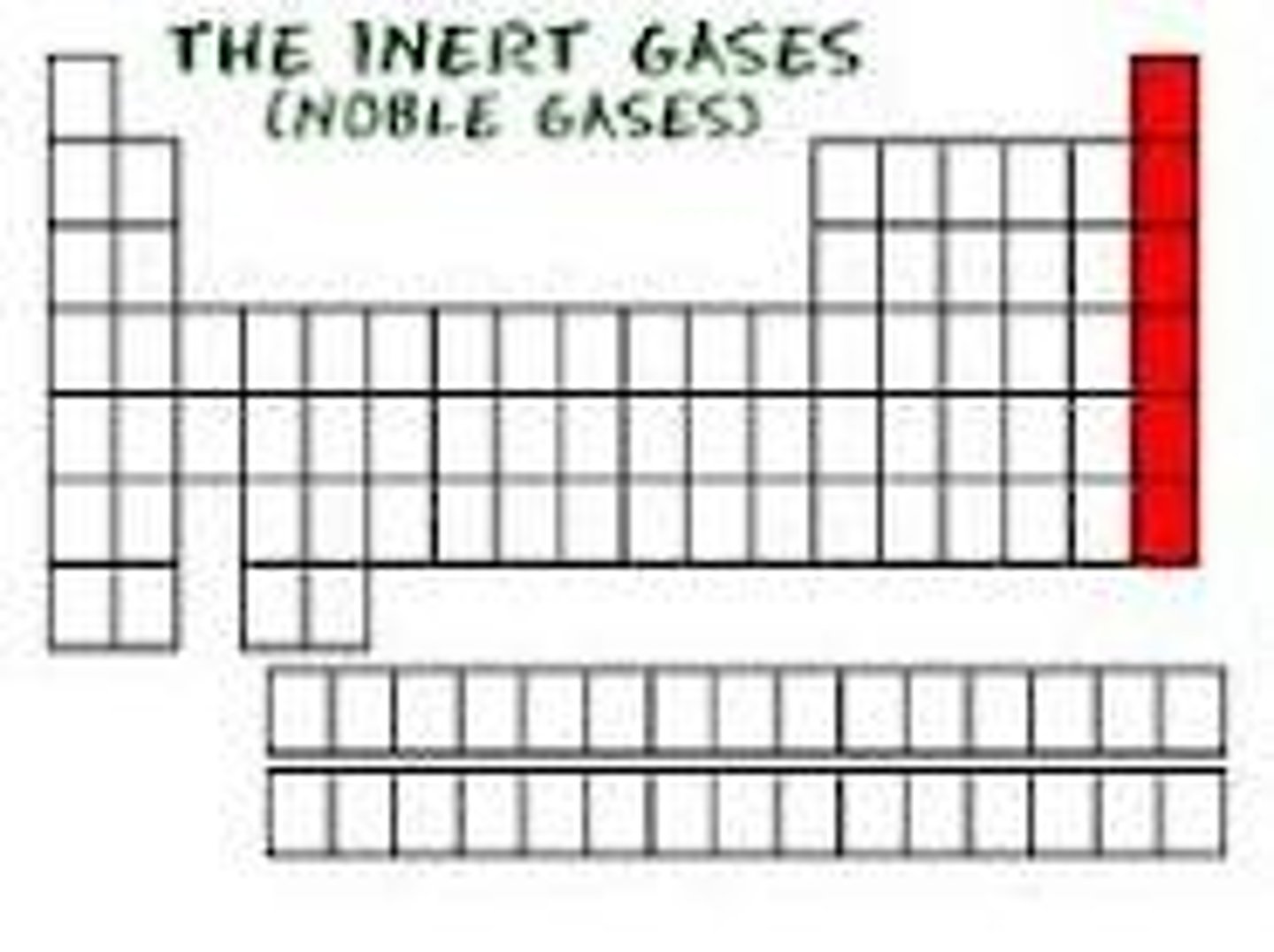
Chemical properties of Group 0
Inert
Electron structure of group 0
All have full shells of electrons and are therefore very stable
Inert
Very unreactive
Physical properties of group 0
All colourless gasses at room temperature
Pattern in boiling point down group 0
Boiling point increases down the group
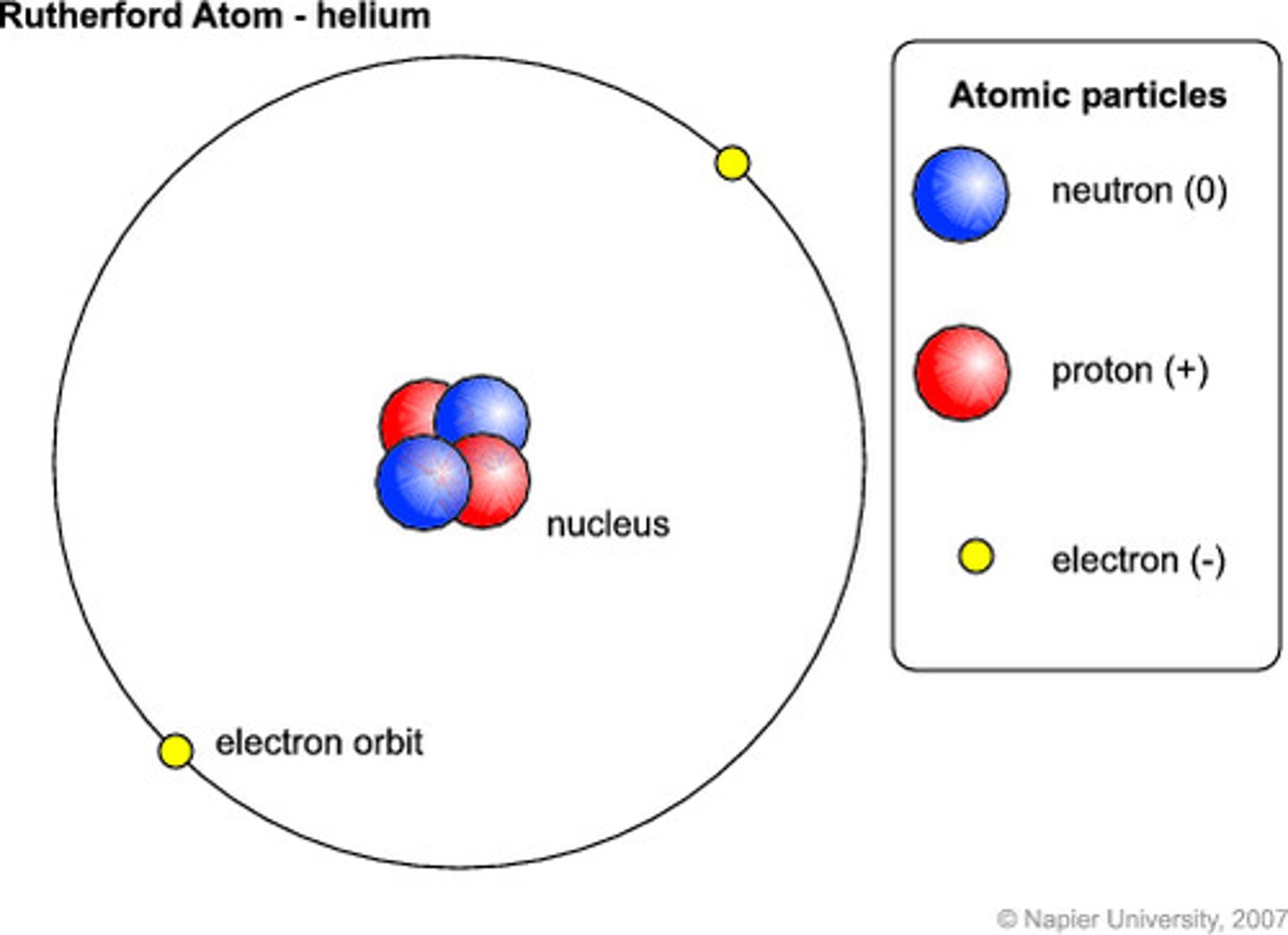
Electron structure of He
2
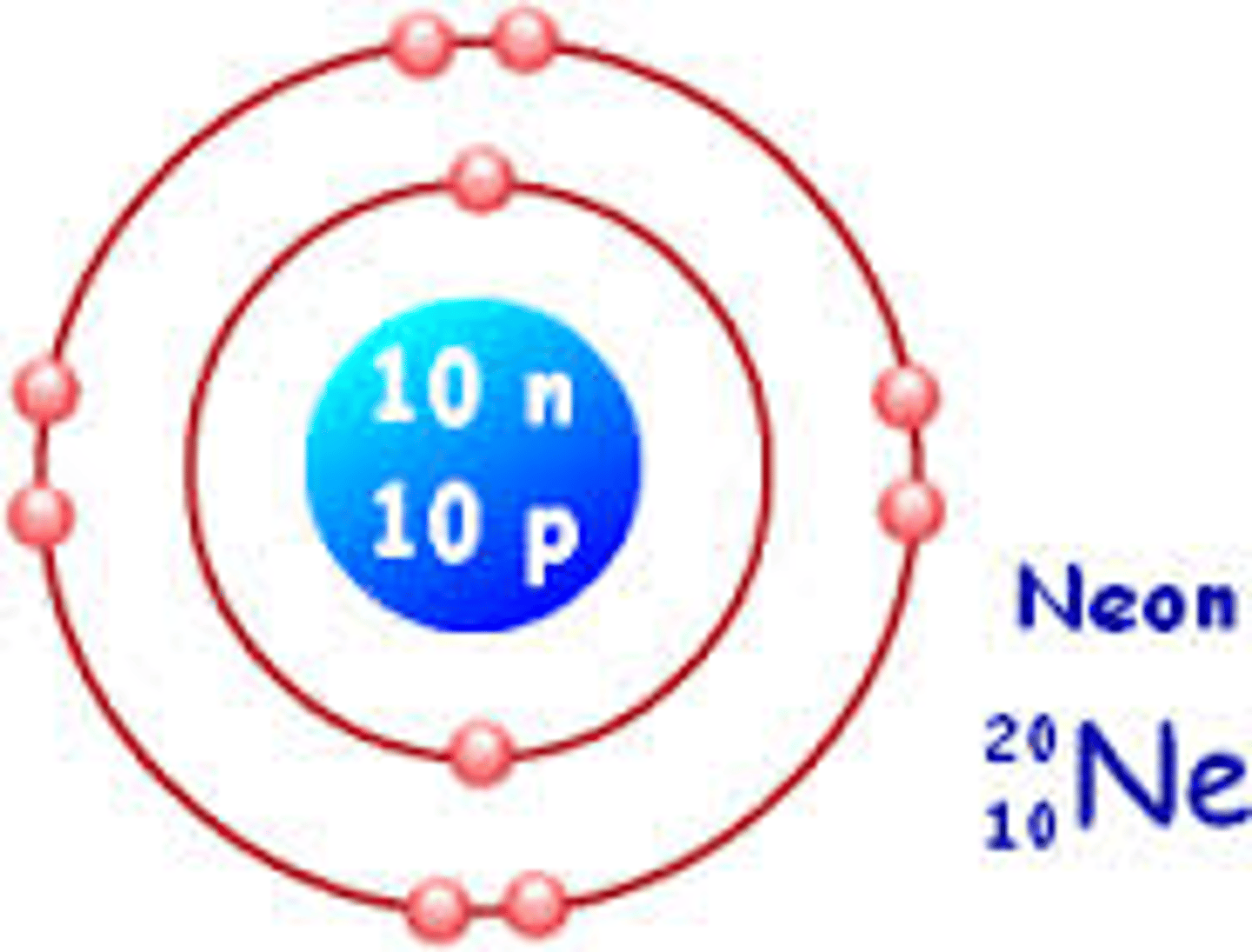
Electron structure of Ne
2,8
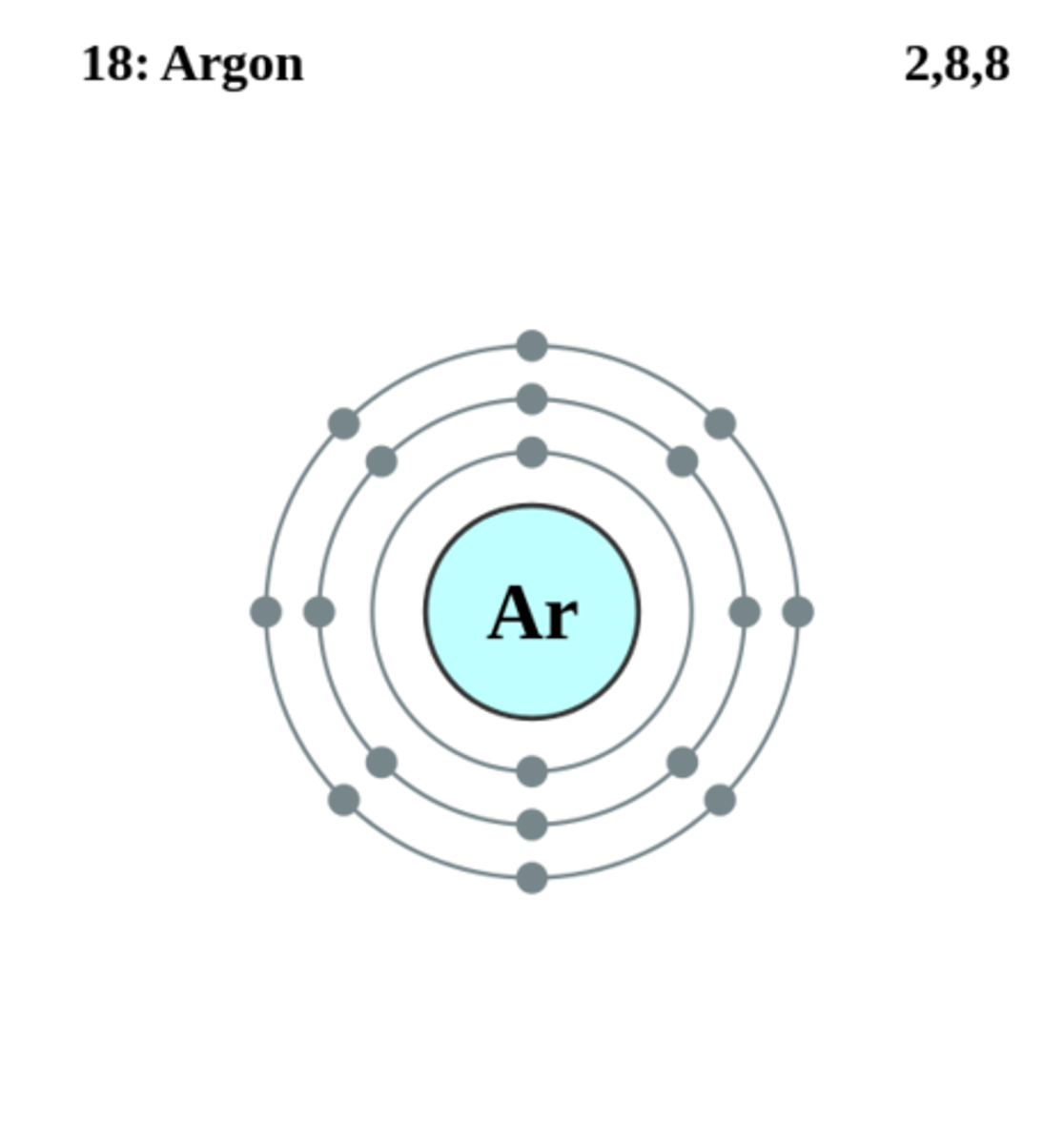
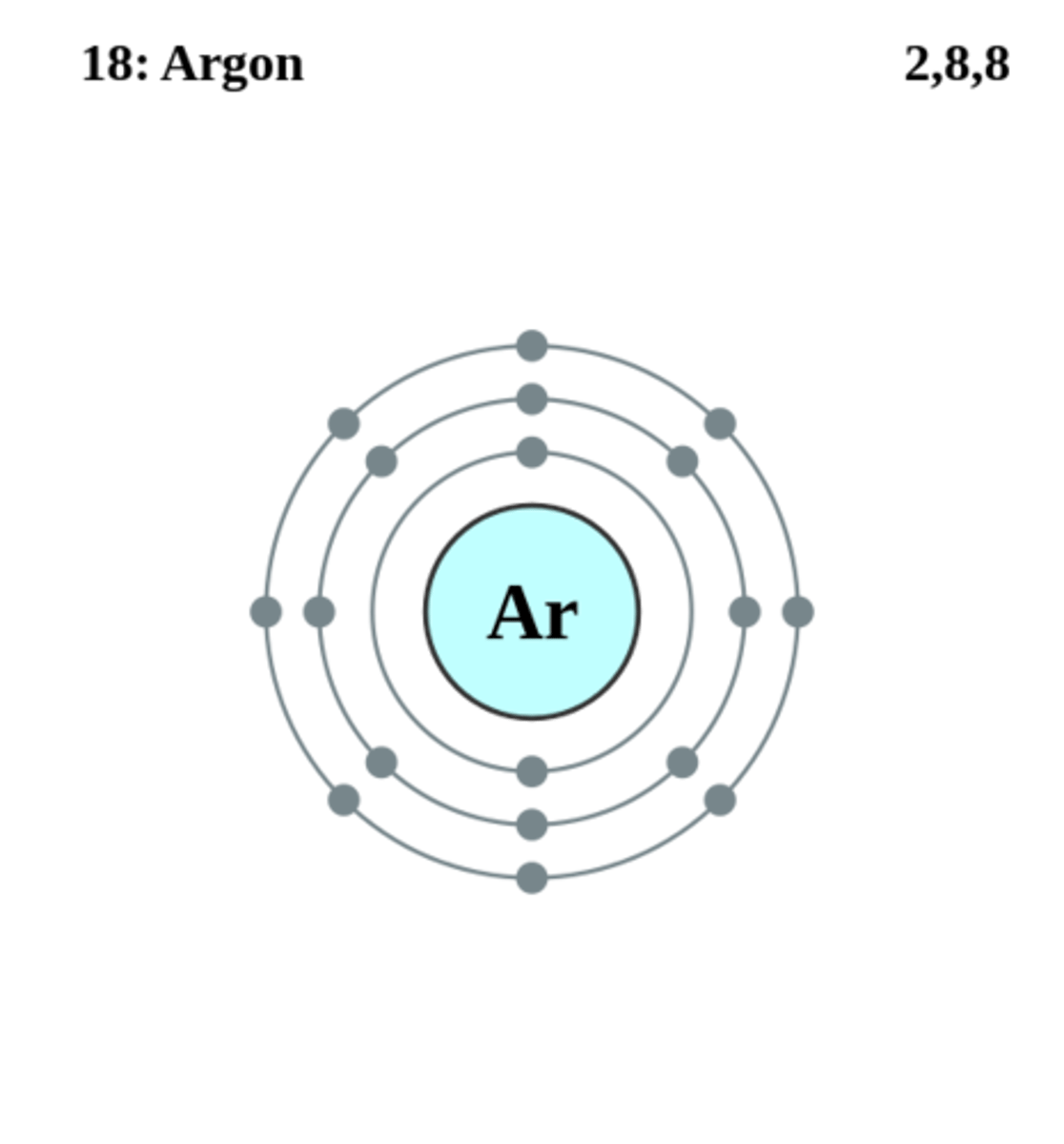
Electron structure of Ar
2,8,8
Monatomic
Made only of single atoms
Chemical structure of group 0
monatomic
Uses of helium
Balloons and airships, because it floats on air
Uses of Argon
In filament bulbs and shield gas in welding because it is inert and more dense than air
Uses of krypton/
Used in light bulbs as it is inert and stops the filament burning away