Chemistry Chapter 5 VESPR Notation & Polyatomic Ions
1/58
Earn XP
Description and Tags
AHHHHHHHHHHHHHHHHHHHHHHHHHHH
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
59 Terms
What does the A stand for in AXE
central atom structure
What does the X stand for in AXE
terminal atoms
What does the E stand for in AXE
lone pairs of electrons
What is the VESPR notation for a linear shape (0 lone pairs)
AX2
What is the VESPR notation for a trigonal planar shape (0 lone pairs)
AX3
What is the VESPR notation for a trigonal planar shape (1 lone pair)
AX2E
What is the VESPR notation for a tetrahedral shape (0 lone pairs)
AX4
What is the VESPR notation for a tetrahedral shape (1 lone pair)
AX3E
What is the VESPR notation for a tetrahedral shape (2 lone pairs)
AX2E2
What is the VESPR notation for a trigonal bipyramidal shape (0 lone pairs)
AX5
What is the VESPR notation for a trigonal bipyramidal shape (1 lone pair)
AX4E
What is the VESPR notation for a trigonal bipyramidal shape (2 lone pairs)
AX3E2
What is the VESPR notation for a trigonal bipyramidal shape (3 lone pairs)
AX2E3
What is the VESPR notation for an octahedral shape (0 lone pairs)
AX6
What is the VESPR notation for an octahedral shape (1 lone pair)
AX5E
What is the VESPR notation for an octahedral shape (2 lone pairs)
AX4E2
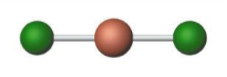
Name the molecular model (name, # of lone pairs):
Linear, 0 lone pairs
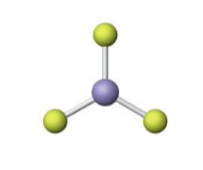
Name the molecular model (name, # of lone pairs):
Trigonal planar, 0 lone pairs
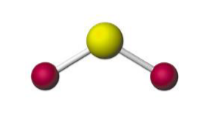
Name the molecular model (name, # of lone pairs):
Trigonal planar, 1 lone pair
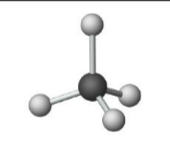
Name the molecular model (name, # of lone pairs):
Tetrahedral, 0 lone pairs
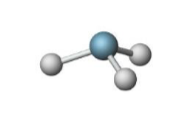
Name the molecular model (name, # of lone pairs):
Tetrahedral, 1 lone pair
Name the molecular model (name, # of lone pairs):
tetrahedral, 2 lone pairs
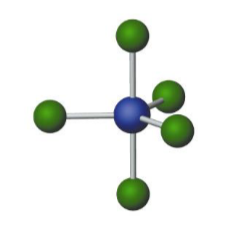
Name the molecular model (name, # of lone pairs):
Trigonal bipyramidal, 0 lone pairs
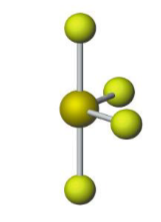
Name the molecular model (name, # of lone pairs):
Trigonal bipyramidal, 1 lone pair
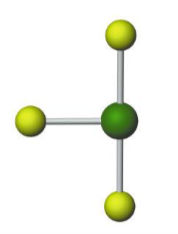
Name the molecular model (name, # of lone pairs):
Trigonal bipyramidal, 2 lone pairs
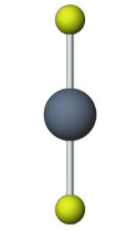
Name the molecular model (name, # of lone pairs):
Trigonal bipyramidal, 3 lone pairs
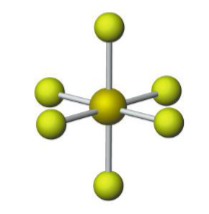
Name the molecular model (name, # of lone pairs):
Octahedral, 0 lone pairs
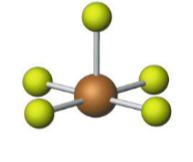
Name the molecular model (name, # of lone pairs):
Octahedral, 1 lone pair
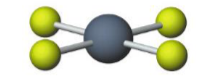
Name the molecular model (name, # of lone pairs):
Octahedral, 2 lone pairs
What is the name of OH-
Hydroxide
What is the name of NH4+
Ammonium
What is the name of NO3-
Nitrate
What is the name of NO2-
Nitrite
What is the name of ClO4-
Perchlorate
What is the name of ClO3-
Chlorate
What is the name of ClO2-
Chlorite
What is the name of ClO-
Hypochlorite
What is the name of (CO3)2-
Carbonate
What is the name of HCO3-
Hydrogen carbonate (bicarbonate)
What is the name of CN-
Cyanide
What is the name of C2H3O2-
Acetate
What is the name of (SO4)2-
Sulfate
What is the name of HSO4-
Hydrogen sulfate (or bisulfate)
What is the name of (SO3)2-
Sulfite
What is the name of HSO3-
Hydrogen sulfite (or bisulfite)
What is the name of (PO4)3-
Phosphate
What is the name of (HPO4)2-
Hydrogen phosphate
What is the name of H2PO4-
Dihydrogen phosphate
What is the name of (PO3)3-
Phosphite
Which nonmetals are found in polyatomic ions
hydrogen, nitrogen, chlorine, carbon, sulfur, phosphorus
What does VESPR stand for
Valence-Shell Electron-Pair Repulsion Theory
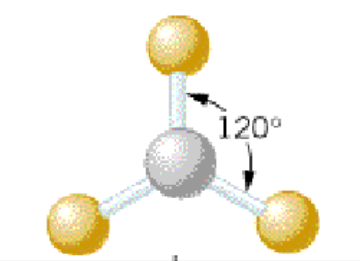
Name the molecular model:
Trigonal planar
True or false: Polar molecules are asymmetric
true
Define hybrid orbitals
mixing atomic orbitals to create new orbitals of equal energy (degenerate orbitals)
Define hypervalent molecules
Elements which have more than an octet
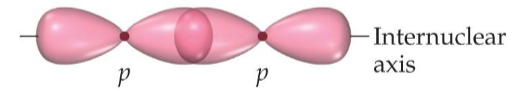
Name the structure:
Sigma bond
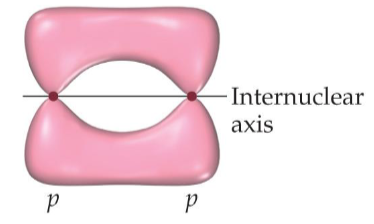
Name the structure:
Pi Bond
What are the characteristics of sigma bonds
head to head overlap and cylindrical symmetry of electron density about the internuclear axis
What are the characteristics of pi bonds
side to side overlap and electron density above and below the internuclear axis