BIOC 202 - Oxidative phosphorylation
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
49 Terms
what does oxidative phosphorylation do?
forms ATP as a result of the transfer of e- from NADH & FADH2 to O2 by e- carriers.
the electrons attached to NADH & FADH2 have what?
high transfer potential (electron motive force)
what is the purpose of the electron motive force (EMF)?
to be harnessed by the electron transport chain (ETC) to transfer protons out of the mitochondrial matrix, thru the inner mitochondrial membrane (IMM), and into the intermediate space (IMS). The resulting electrochemical gradient forms a proton motive force (PMF)

what can the proton motive force (PMF) be used for?
to form ATP (a chemical w/ high phosphoryl transfer potential) by ATP synthase
where is the ETC and ATP synthase located?
inner mitochondrial membrane (IMM)
what is the IMM impermeable to?
small mlcs and ions
what is the outer mitochondrial membrane (OMM) permeable to?
small mlcs and ions as it contained pores thus is considered leaky
how can things be moved across the IMM?
thru transporters
what is the inner mitochondrial space (IMS) similar to?
cytosol
what are 3 ways that electrons can be transferred?
free e-
H+
Hi- (hydride ions)
what is standard reduction potentials (E0’)?
the measurement of mlcs tendencies to accept e- in volts
the more positive the E0’, the _____ the mlcs affinity for e-
higher
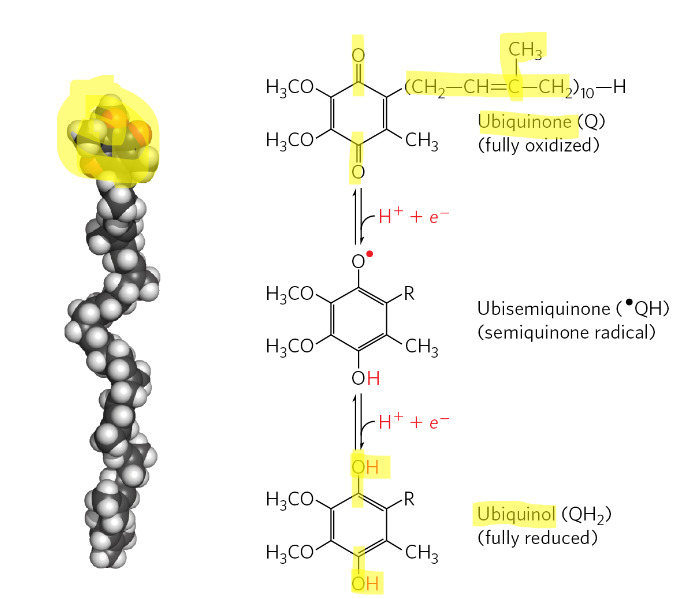
what is the Nerst equation?
relating delta G0’ to E0’
n = number of electrons
F = Faraday’s constant (96.5kj/V x mol
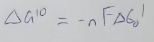
how to calculate delta E0’ when u have the E0’ for the e- acceptor and donor?
Delta E0’ = E0’(acceptor) - E0’(e- donor)
per NADH, how many ATP are formed?
2.5
In the process of transferring e- thru a series of e- carriers, increasing E0’ until they reach O2, what happens to protons?
they are moved into the IMS
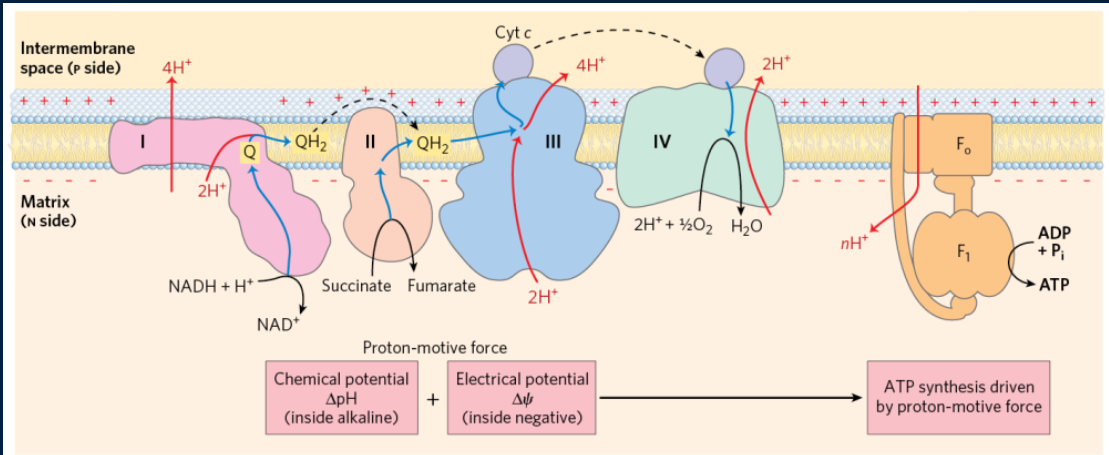
what is the ETC composed of and what does it contain?
composed of 4 major complexes, and containing multiple protein subunits and e- carriers. There are also 2 e- carriers that act as shuttles, moving e- from complex to complex.
in the ETC, what is complex 1 called?
NADH-Q Oxidoreductase
Explain what happens in complex 1 of the ETC
The protein is going to accept 2e- from NADH (oxidizing it to NAD+), which are going to be transferred to FMN and then a series of 4Fe-4S clusters, and then finally, to coenzyme Q (ubiquinone), reducing it to QH2 (ubiquinol). This results in enough energy to pump out 4 H+ out of the mitochondrial matrix and into the IMS

what is the net rxn of complex 1?
NADH (matrix) + 5H+ (matrix) + Q → NAD+ (matrix) + 4H+ (IMS) + QH2
how do iron-sulfur clusters transfer electrons?
by reducing the bonds between the Fe-S as electrons are stored in the bonds between S-H

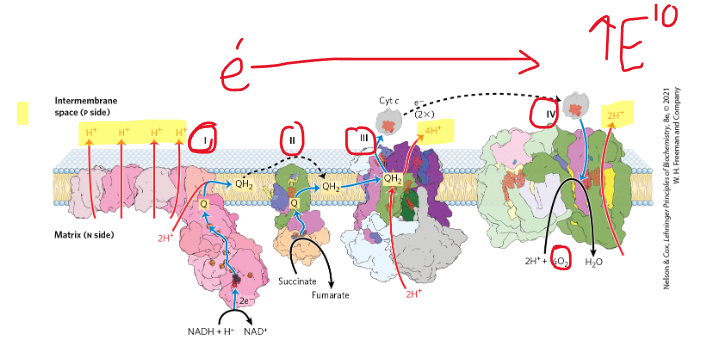
what is the name of complex 2 in the ETC?
succinate Q reductase
what is unique about complex 2 compared to the other complexes in the ETC?
no protons are transferred through this complex so e- from FADH2 do not move as money H+ as NADH across the IMM
what Krebs cycle enzyme is also a part of complex 2?
succinate dehydrogenase
Explain what happens in complex 2 of the ETC
electrons are transferred from succinate to fumarate, then to FAD (reducing it to FADH2), then to the succinate Q-reductase, flowing through a series of Fe-S complexes, and then finally to Q - forming QH2

do electrons from NADH pass thru complex 2? why or why not?
no bc complex 1 and complex 2 are not connected
where are the electrons used in complex 2 coming from?
from FADH2 of the Krebs cycle
draw out the simplified version of complex 2
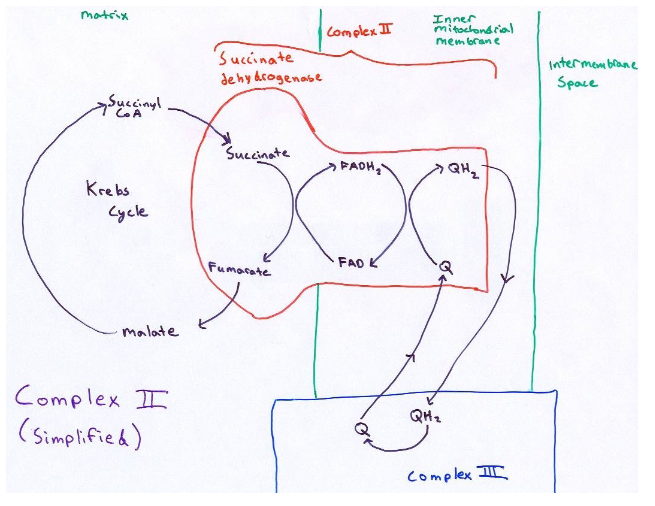
what is ubiquinone/ubiquinol? where are they located? what do they contain?
they are small hydrophobic molecules located in the IMM that act as shuttles, moving e- from complex 1 and 2 to complex 3 (e- don’t pass thru complex 1 to 2). They contain a repeating isoprenoid tail
how many repeating isoprenoid tails on ubiquinone/ubiquinol are there in humans?
10 (CoQ10)
how is ubiquinone (Q) reduced to ubiquinol (QH2)?
by accepting 2 H+ and 2 e-
Q + 2e- + H+ → QH2
what is complex 3 of the ETC called?
Q-cytochrome C oxidoreductase
what 2 components does complex 3 consist of?
2Fe-2S clusters
2 key cytochromes: cyt b & cyt c
what hemes do cyt b & cyt c contain?
cyt b → heme bL & heme bH
cyt c → heme c
what is a cytochrome?
an electron transferring protein containing one or more hemes
explain the electron flow in complex 3
electrons flow from QH2 (generated from complexes 1 and 2) to 2Fe-2S cluster, to heme c, and finally to heme L (in cyt c). But, when QH2 docks, 1 e- follows the flow mentioned in the sentence before, but the other electron goes to heme b’s and participates in the Q cycle. Then it follows the first e to the 2Fe-2S cluster
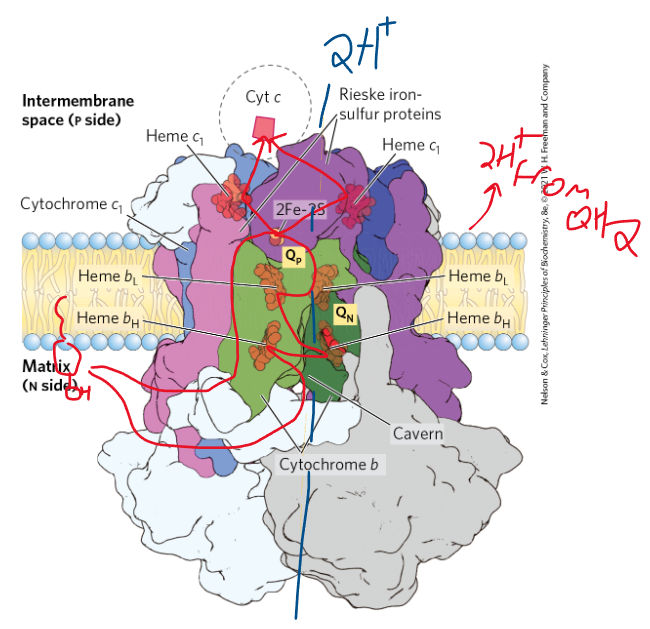
the Q-cycle is the process of….
moving electrons and pumping H+ thru complex 3 (if u have all electrons going straight to the 2Fe-2S cluster, no protons will be pumped out)
what is the net equation of complex 3?
QH2 + 2 cyt c (ox) + 2 H+ (mat) → 2 cyt c (red) + Q + 4H+ (IMM)
what is cytochrome c? what does it do?
a water soluble protein containing a covalently linked heme. It carries 1e- from complex 3 to complex 4 and rolls along the surface of the IMM on the IMS side
What is complex 4 called?
cytochrome c oxidase
Explain what happens in complex 4
complex 4 carries out the final reduction of oxygen using electrons from cyt C. It requires 4 electrons to completely reduce O2 to H2O and in the process, 4 H+ are pumped into the IMS. O2 binds to heme a3 and then bridges btwn heme a3 & CuB
what 2 components does complex 4 contain?
2 cytochromes - Cyt a & Cyt a3
2 copper centers - CuA & CuB
describe the electron flow in complex 4
electron flow is from heme c (cyt c) → CuA → heme a → finally to heme a3/CuB and onto O2

What is the net rxn of complex 4
2 cyt C (red) + 4 H+ (mat) + 1/2O2 → 2 cyt C (ox) + 2 H+ (IMS) + H2O
2e- from NADH → ?
2e- from FADH2 → ?
10 H+ pumped into IMS (complex 1, 3, 4)
6 H+ pumped into IMS (complex 3, 4)
what is complex 4 responsible for?
preventing the release of partially reduced O2 (O2- = superoxide, O2 2- = peroxide). Partially reduced oxygens are known as reactive oxygen species (ROS) and they can damage DNA and proteins.
draw the ETC
What complication can occur in the ETC?
some mlcs can bind to various e- carriers and block e- transfer. The e- carriers before the block become reduced and the e- carriers after the block will become oxidized. These mlcs (e.g. N3-, CO, CN) bind to the heme in cyt a3 in complex 4 and prevent o2 binding and e- flow to o2. This blocks the ETC, preventing generating of H+ gradient, ATP synthase slows/stops, no ATP synthesis, and u can die