Quantum chemistry: History and concepts
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms

A-planck’s constant, h

A- the born-Oppenheimer approximation

D- the product of the two uncertainties is > or equal to h/4pi

A- the Pauli exclusion principle

D- kinetic energy is a function of frequency and the current is a function of intensity of the light incident light

D- total energy operator

D
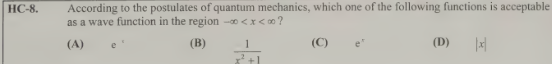
B- 1/ x²+1

A- 2px is not an eigenfunction of L, thus there is no definite value
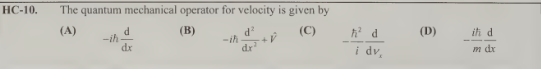
D

A- the schrodinger equation is solved by assuming the nuclei are stationary

B- may be simultaneously determined with unlimited precision
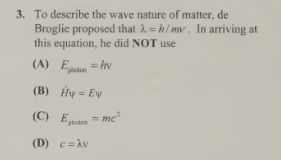
B-

D- integral 1* and 2 = 0

A- physically acceptable wave function must be finite, single values, and continuous; and have a continuous first derivative

C-separating regions of different sign for a wave function

B- e^ax

C- a sum of 2 degenerate eigenfunctions for an operator is still an eigenfunction for that operator

A- integral (x)* px (x)dx

D- e^-x over the range 0 to infinity

A- the intensity of the light hitting the metal