Grade 10 Academic Science Chemistry
1/22
Earn XP
Description and Tags
Chemistry Test Flashcards
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
Where does the 7 + 1 rule start and end
Starts at Nitrogen ends at iodine + hydrogen
Name all 5 prefixs, and include which prefix is never used for the first element
mono, di, tri, tetra, penta (mono is never used)
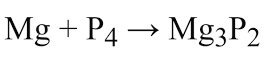
Name both sides of the arrow
reactants - products
Metals are on which side of the periodic table
left side
Non metals are on which side of the periodic table
right side
Descriptors of metals
Metals
Shiny (lustrous)
Malleable (can be hammered into sheets)
able to be stretched into wires)
Good conductors of heat & electricity
Usually solid
Description of non mentals
Dull (not shiny)
Brittle (breaks/crumbles if stretched or hammered)
Poor conductors (insulators)
Can be solid, liquid, or gas at room temp
Name all 4 types of chemical reactions
Synthesis
Decomposition
Single
Double
Define all 4 types of chemical reactions
Synthesis = A+B=AB
Decomposition= AB = A + B
Single = A + BX = AX + B
Double = AX + BY = AY + BX
Combustion → Hydrocarbon + O₂ → CO₂ + H₂O (if complete)
skeleton equation is the same as
unbalanced equation
what does pH stand for?
powers of hydrogen
what is the purpose of pH
measures the strength of an acid or base
at what number does a chemicals pH’s levels have to be for it to be neutral
7
For a chemical to be neutral it needs to have a equal number of two elements, what are they
hydroxide (OH) and hydrogen ions
Ph more than seven =
base, stronger, larger
Ph less than seven =
acidic/very acidic
Acid indicator
sour, turns blue, reacts with chalk/calcium carbonate, produces CO2, reacts with zinc & other metals to produce hydrogen gas
Base
Bitter, slippery, changes the color of red litmus, corrosive conducts electricity
Neutralization reactions are =
double displacement
Neutralization equations produce
water + salt
Neutralization equations have a ____ and ____ as reactants
base and acid
In a bohr rutherford diagram the atomic number is the same as
protons/electrons
Atomic mass - atomic number = ?
Neutrons