S1.3 Electronic Configuration
1/30
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
Define the electromagnetic spectrum
The electromagnetic spectrum (EMS) is a spectrum of wavelengths that comprise the various types of electromagnetic radiation
It is arranged from increasing energy and frequency but a decrease in wavelength
Includes :
Gamma
X-rays
Ultra violet
Infrared
Microwaves
Radiowaves
What is the difference between emission line spectrum and continuous line spectrum?
A continuous spectrum in the visible region contains all the colours of the spectrum
However, a line spectrum only shows certain frequencies
This tells us that the emitted light from atoms can only be certain fixed frequencies - it is quantised (quanta means 'little packet')
Describe the relationships between
→ E inversely proportional to 1/λ
Energy and frequency are indirectly proportional to wavelength
—> c = vλ
The relationship between speed and wavelength and frequency
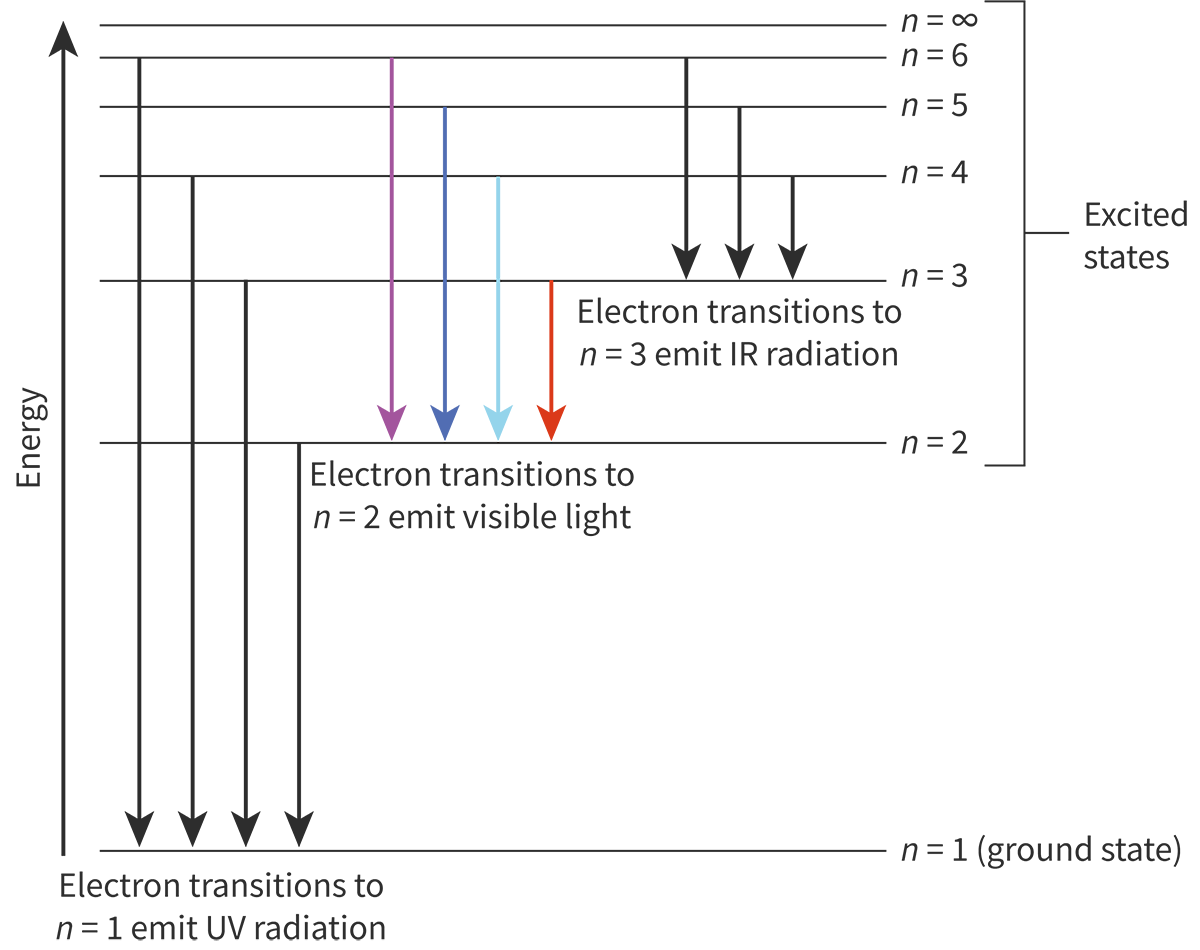
Describe and explain the hydrogen emission spectrum
when hydrogen atoms are subjected to a particular amount of energy the electrons will absorb this energy and be lifted to a higher energy level, making them unstable and they will emit that same amount of energy, which relates to a certain light.
Stable electrons are on the ground, but excited ones that have absorbed energy are uplifted higher and become unstable, like being lifted by people, where eventually you have to come back down.
How does the emission spectra relate to other elements?
All elements in the gaseous state can go through what hydrogen does
When they release a certain amount of energy, they emit a certain color of light
This allows them to be identified
Hydrogen is just the most abundant element
How does the emission spectra relate to the quantization of energy?
The precise lines in the line emission of an element have specific wavelength.
The shorter the wavelength, the higher the energy, the longer the wavelength, the lower the energy.
Electrons jump to one energy level to another and fall back to ground state.
This is the transition responsible for color.
Define Quantisation
the idea that electromagnetic radiation comes in quanta (like amazon parcels).
This way we can quantify the amount of energy.
They come as a whole.
Once packet of energy (quantum) or photon is released for energy transition.
What is Plank’s constant?
Ephoton = hv
→ h = 6.63 x 10^-34 Js
→ E = hv = hc/λ
The energy of light, (Ephoton of light) is related to its frequency, v by Plank’s Constant.
→ Ephoton is inversely proportional to frequency.
Who is Neil Bohr?
suggested that specific energies could be explained if the electrons could only move to specific orbits around the atom. And that these orbits have specific energies.
The Bohr Model : first level holds two electrons, while the second holds eight and etc.
Define Spectroscopy and the dual properties of light
The study of interaction between matter and light is called spectroscopy.
When light is described by frequency, it is a wave and when described by energy —> individual particles, as photons then it is a particle. This is the dual property of light.
When light diffracts or spreads out, it is equal to a wave and scattering of electrons, when light hits a metal substance, making it a particle.
How many orbitals are in each main and sub shell?
n = 1 (1) : 1s
n = 2 (2) : 2s, 2p
n = 3 (3) : 3s, 3p, 3d
n = 4 (4) : 4s, 4p, 4d, 4f
How many electrons can each orbital hold?
1s - 2 = 2
2s, 2p - 2,6 = 8
3s, 3p, 3d - 2, 6, 10 = 18
4s, 4p, 4d, 4f - 2, 6, 10, 14 = 32
What is the actual arrangement of the orbitals?
1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s etc
The Aufbau principle
states that when adding electrons to an atom, the lower energy orbitals must be filled first.
The Pauli exclusion principle
states that an atomic orbital can only hold two electrons and they must have opposite spins.
Hund’s rule
states that when we have
degenerate orbitals (orbitals of the same energy) then each orbital is filled with a single electron before being doubly occupied.
Configuration of Transition Metals
Instead of writing the entire thing, we put the noble gas that is aligned with the metal and any excess electrons afterwards.
Define Ionisation Energy
This is the energy required to remove an electron from a neutral gaseous atom or molecule in its ground state.
Define First Ionisation Energy
energy required when one mole of gaseous atoms form one mole of gaseous ions with a single positive charge.
Define Second Ionisation Energy
This is the energy where one mole of gaseous ions with a single positive charge forms one mole of gaseous ions with a double positive charge.
Describe Convergence in relation to ionisation
At the limit of convergence, the lines blur to form a continuum and beyond this line, the electron is outside of the atom and ionisation has occurred
What are factors that affect ionisation energy?
1. Attraction of the nucleus
2. The distance of the electrons from the nucleus
3. Shielding of the attraction from the nucleus
Why does the ionisation drop as you go down the group?
This is because the electrons are found in shields further from the nucleus and are more shielded, meaning they are more attracted to the other atom
Why does the ionisation increase as you go across the period?
Number of protons increase, making the effective attraction of the nucleus greater
this means noble gasses are always at the peak
Why is there a drop between group 0 and group 1 elements?
The electrons in group 1 are arranged with a new shell increasing their shielding
this is easier to remove so ionisation energy decreases
Why is there a drop between group 2 and group 3?
The electrons in the 3p sub shell are slightly higher in energy so they are easier to remove
They are also shielded by the 3s electrons
Why is there a small drop from P to S?
when the second electron is added to a 3p orbital there is a slight repulsion between the two negatively charged electrons and the second electron is easier to remove
Why are successive ionisation energies bigger than the first?
Because when the first ionisation energy occurs, the atom has become a positive ion, having a higher pull on the rest of the electrons
So more energy is needed for the electrons to be able to become ionized
Why is there a bigger jump between 4th and 5th ionisation energies?
fifth electron is a inner main shell closer to the nucleus and therefore attracted much more strongly by the nucleus than the fourth
it also does not have any shielding by inner complete shells
Calculating Ionisation Energies
E = hv = hc/lambda
Describe the shape of s and p orbitals
S = sphere
P = dumbell