quiz #1: aquaporins and insulin
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
when proteins don’t work ___, typically occurs
disease
what parts of the membrane are polar / nonpolar?
polar phospholipid head (exterior), non-polar phospholipid tails (interior)
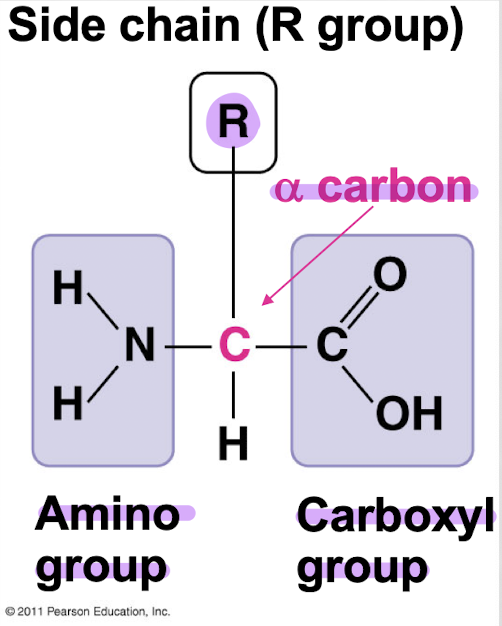
what are the 3 components of an amino acid?
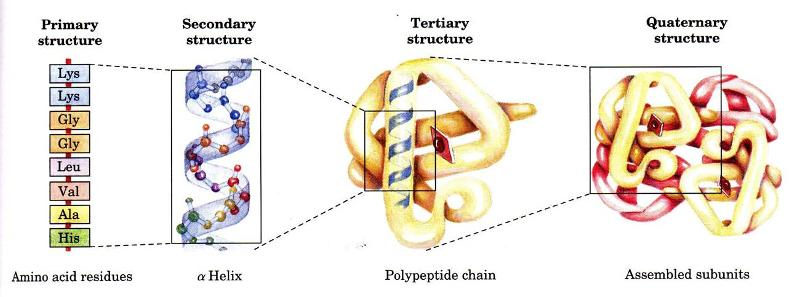
what are the different levels of protein structure?
The primary structure consists of amino acids connecting through peptide bonds.
The secondary structure consists of the amino acid backbone bonding through hydrogen bonds.
The tertiary structure consists of R groups (side chains) that bond together and fold into a 3D structure. This level consists of several possible bonds, including H bonds, disulfide bonds, ionic bonds, and van der Waals forces.
The quaternary structure consists of multiple tertiary structures bonding together with one another. This level consists of the same interactions as the tertiary level.
how to determine if an amino acid is polar or nonpolar?
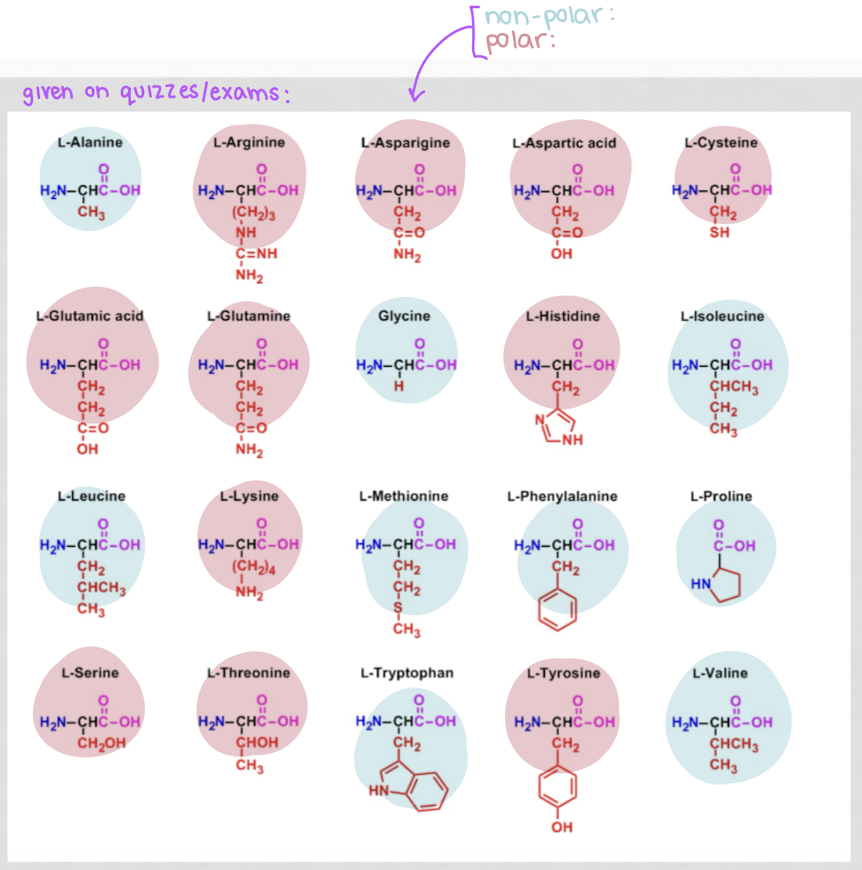
with the side chain, its polar if it:
has an oxygen
has more than 1 electronegative atom in the R group
what are the different types of view models?
space-filling model, ribbon model, ball-and-stick model
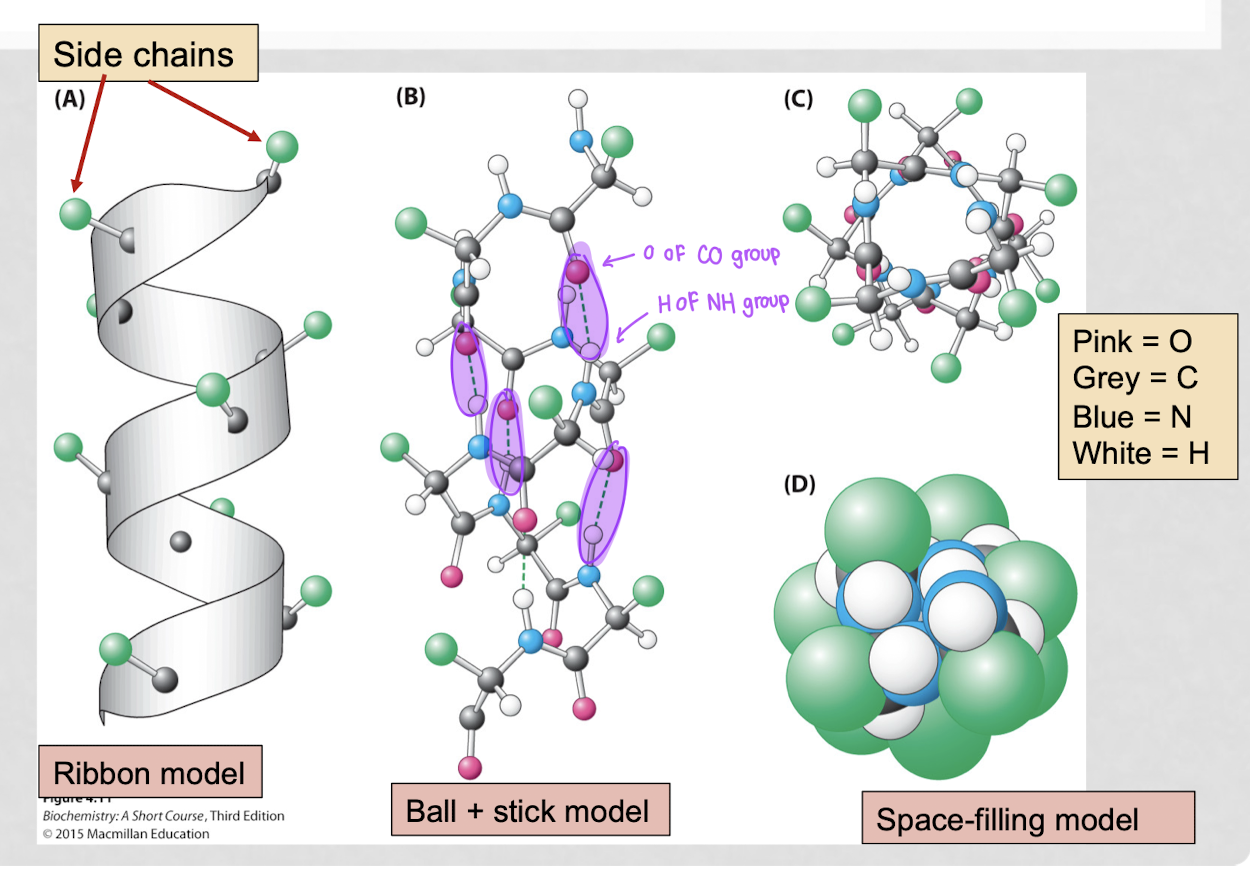
what is the space-filling model?
represents atoms as spheres scaled to their van der Waals radii
best for: visualizing molecular shape, size, steric hindrance, and intermolecular interactions
what is the ribbon model?
depicts the structural backbone of macromolecules (proteins, DNA)
best for: understanding secondary/tertiary structures, folding patterns, and functional regions
what is the ball and stick model?
shows atoms as balls and bonds as sticks
best for: analyzing molecular geometry, bond angles, bonding, and functional groups
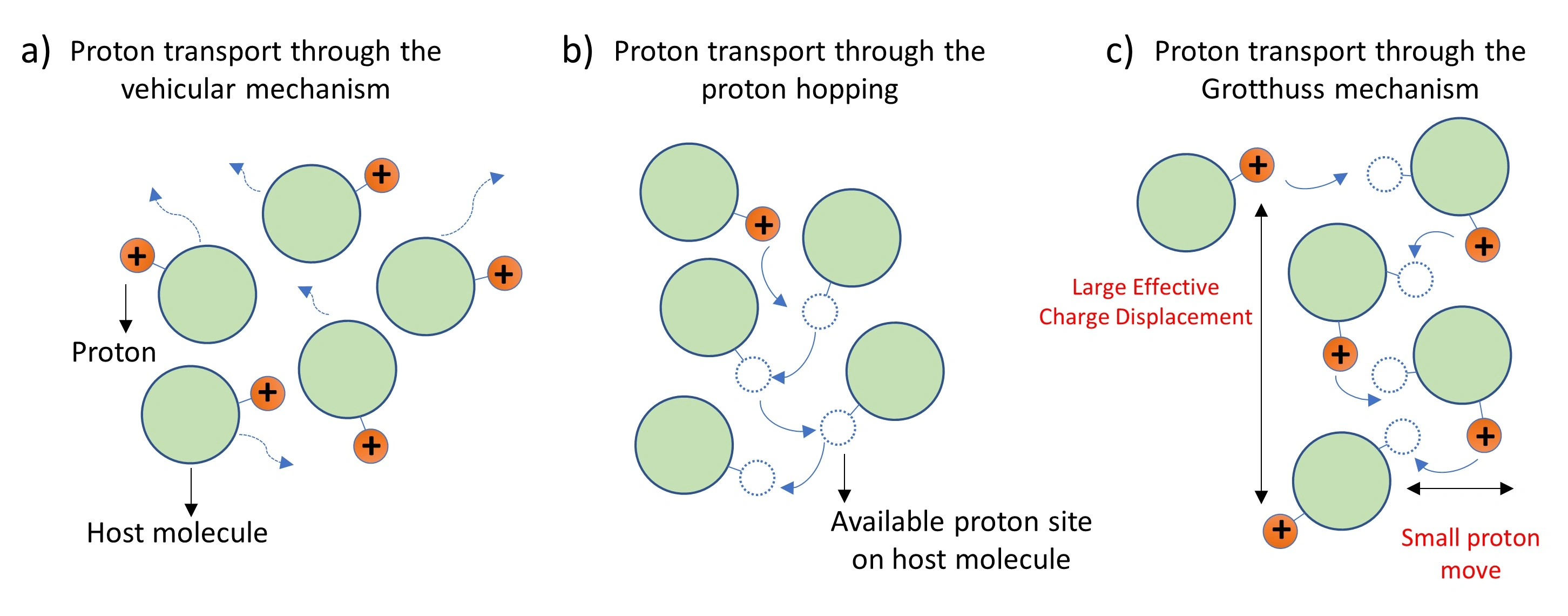
what is the grotthuss mechanism?
explains how protons (H⁺) are transferred through water or other hydrogen-bonded networks. instead of moving as individual protons, the transfer happens through a relay process:
a proton hops from one water molecule to another, forming a hydronium ion (H₃O⁺).
this causes a chain reaction where protons are passed along the hydrogen bond network.
water molecules continuously exchange roles as proton donors and acceptors.
is why protons diffuse faster in water than other ions
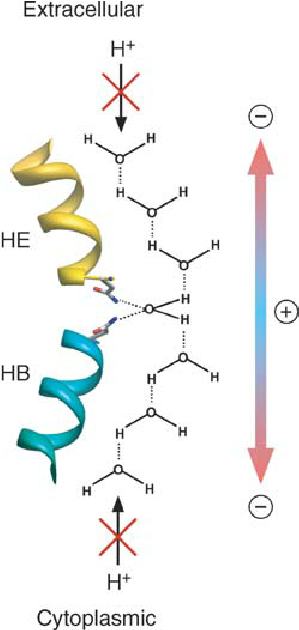
what is the NPA motif?
a conserved sequence found in aquaporins and similar membrane channels. It plays a key role in:
structuring the channel: helps form the narrow pore that selectively allows water molecules to pass through.
blocking ions: Prevents the movement of protons and other charged particles, maintaining the cell's electrochemical balance.
ensures aquaporins are highly efficient and selective for water transport
how does an aquaporin maintain selectivity for water? (3)
1. One way in which aquaporins remain selective is through size restriction. The narrow internal portal going into the intracellular space is small and restricts molecules of a larger size from entering.
2. The second way it remains selective is through electrostatic repulsion. Arginine groups and other positively charged groups are present on the ends of the channel are positive and deter away other positively charged molecules. Negative groups are present in the middle of the channel and deters negatively charged molecules from passing through.
3. The third way is remains selective is through the NPA motif. By Asparagine disrupting the Grotthus mechanism and reorienting water to break water-water hydrogen bonds, this pretvents proton transfer in order to protext the pH, voltage, and other internal cellular functions.
what is the hydrophobic effect?
the tendency of nonpolar substances to aggregate in aqueous solution, minimizing their exposure to water. this phenomenon drives the formation of cellular membranes and protein folding
why do micelles form?
gain of entropy of water
Given that phospholipid tails are nonpolar and thus hydrophobic to water, water molecules order themselves around the tails, decreasing the entropy of the system due to water's limited movement to maintain this hydrogen bonding. The phospholipid tails do not like to interact with water and are attracted to one another through van der Waal's forces, causing them to stabilize in a sphere-like shape through these interactions. By allowing the phospholipid tails to cluster together in a micelle, this minimizes their disruption of water's hydrogen bonding and increases the entropy of the system, making it more favorable.
how do non covalent interactions contribute to protein folding and stability?
Hydrogen Bonds: Stabilize secondary structures.
Ionic Interactions: Form salt bridges between charged side chains.
Van der Waals Forces: Pack atoms tightly in the protein core.
Hydrophobic Interactions: Drive nonpolar residues inward, shielding them from water.
are the following full/partial charges and permanent or temporary?: ion-ion, hydrogen bond, van der waals
ion-ion: full, permanent
HB: partial, permanent
VDW: partial, temporary
why is protein folding spontaneous?
Protein folding is spontaneous because it decreases the system's free energy (ΔG). This occurs through:
Decrease in Enthalpy (ΔH): Formation of stabilizing non-covalent interactions (hydrogen bonds, ionic interactions, van der Waals forces) releases energy.
Increase in Entropy of Water: Hydrophobic residues burying in the protein core release ordered water molecules, increasing the entropy of the surrounding environment.
Together, these factors make the overall ΔG negative, driving the folding process.
what is the pKa?
the pH at which the acid is half-dissociatedin solution, meaning that the concentrations of the protonated and deprotonated forms are equal
how do you make a polypeptide?
take the OH from the carboxy terminal and an H from the amino terminal and make H2O to form a peptide bond
when to protonate / deprotonate?
protonate: pH < pKa
deprotonate: pH > pKa