Chemical Energetics
0.0(0)
Card Sorting
1/12
There's no tags or description
Looks like no tags are added yet.
Last updated 4:02 PM on 10/13/24
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
1
New cards
Standard State
1 bar (=10^5Pa), 1 mol dm^-3, conventionally: 298K (25C)
2
New cards
Standard Enthalpy Change of Reaction
Energy change when molar qty of rxtants specified by chemical eqn rxts to form pdts. (With all rxtants and pdts at standard states.
3
New cards
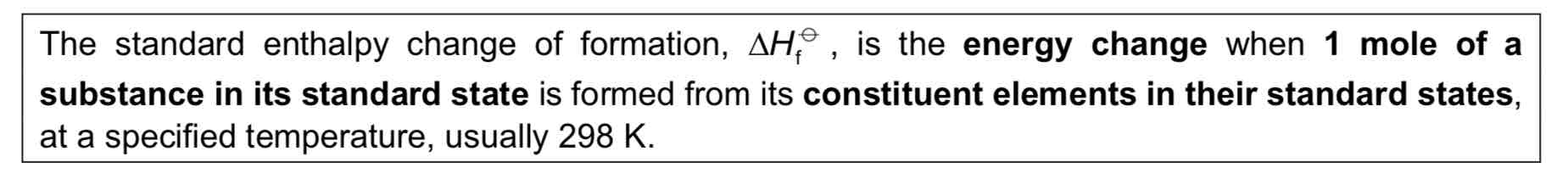
Standard Enthalpy Change of Formation
4
New cards
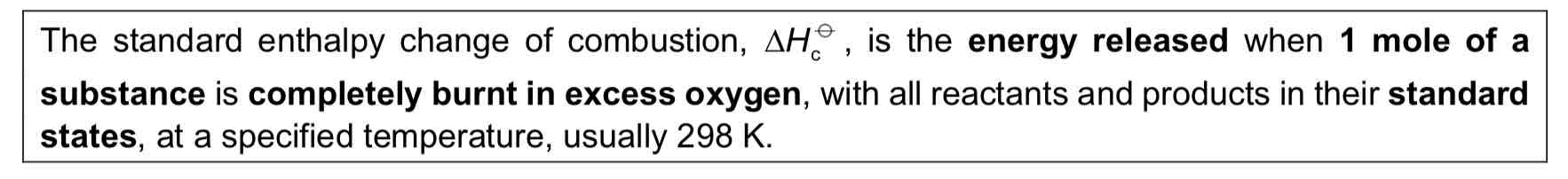
Standard Enthalpy Change of Combustion
5
New cards
Standard Enthalpy Change of Neutralisation

6
New cards
Bond Energy

7
New cards
Standard Enthalpy Change of Atomisation (element)

8
New cards
Standard Enthalpy Change of Atomisation (compound)

9
New cards
Lattice Energy

10
New cards
Ionisation Energy

11
New cards
Electron Affinity

12
New cards
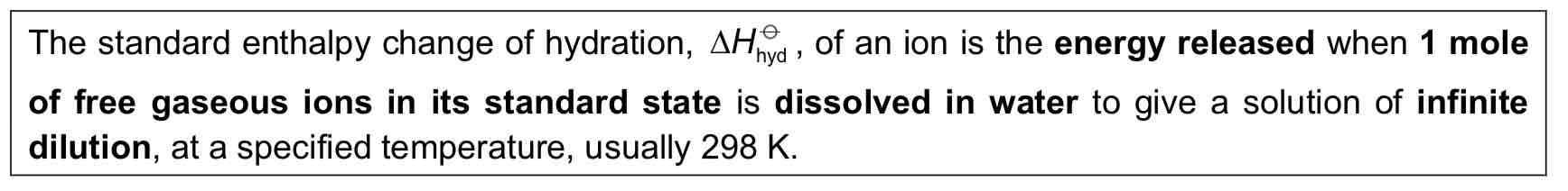
Standard Enthalpy Change of Hydration
13
New cards
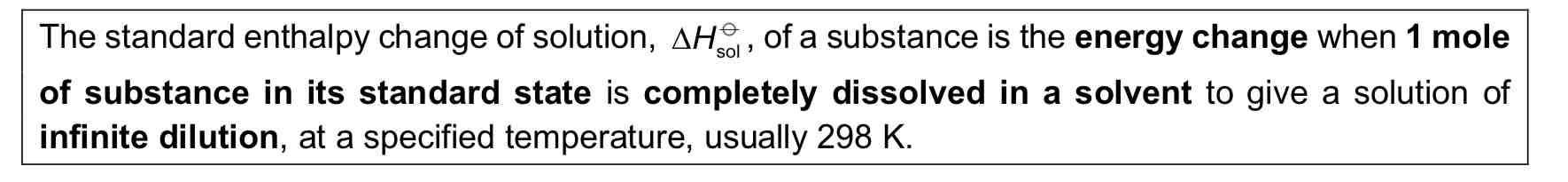
Standard Enthalpy Change of Solution