Biochemistry Chapter 10: Carbohydrate Metabolism II (Aerobic Respiration) ^^^
1/94
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
95 Terms
what is Acetyl-CoA?
key molecule in the crossroads of several metabolic pathways
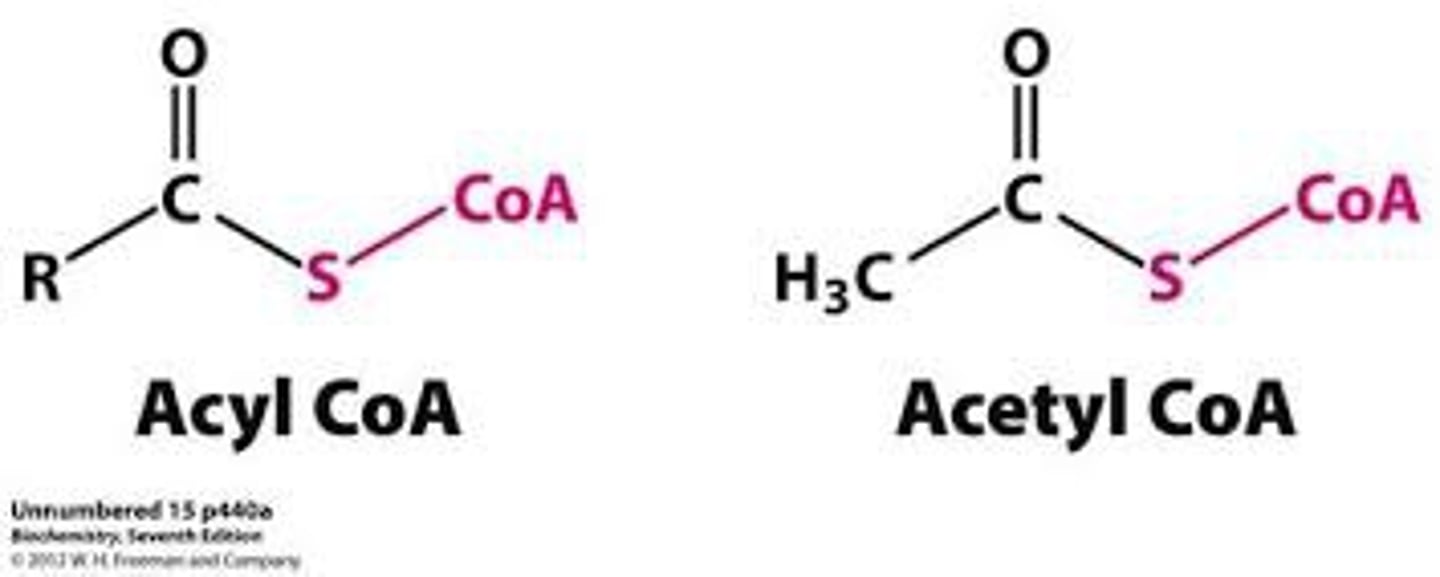
** important organic chemistry note **
*acetyl groups are a type of acyl groups*
- acyl groups are: R1-C (=O)-R2
- acetyl groups are: Me-C (=O)-R
4 methods of forming Acetyl-CoA
1) PDH complex --- Major
2) beta-oxidation --- Major
3) ketone bodies (ketogenic amino acids) --- minor
4) alcohol --- minor
result of all 4 of the previous methods
entrance of Acetyl-CoA into the TCA cycle
PDH complex
- converts 3 carbon pyruvate to 2 carbon Acetyl-CoA (CO₂ is released)
- uses 5 enzymes (w/ coenzymes and cofactors), coenzyme A, and NAD+
- exergonic reaction (spontaneous ; - delta G)
Coenzyme A
is a thiol so when Acetyl-CoA forms, it makes thioester bond
importance of thioester bond
VERY high energy
- can drive citric acid cycle forward when broken
5 enzymes used in the PDH complex
1) pyruvate dehydrogenase (PDH)
2) dihydrolipoyl transferase (dHLT)
3) dihydrolipoyl dehydrogenase (dHLD)
4) PDH kinase
5) PDH phosphatase
which 3 of the 5 enzymes are used for the actual creation of Acetyl-CoA from pyruvate
PDH, dHLT, dHLD
- ALL of these move in their own loop which give/take different molecules to help form products
so then what do PDH kinase and PDH phosphatase do in the PDH complex?
regulate PDH by phosphorylating or dephosphorylating (respectively) it
- ADP activates PDH phosphatase (activates PDH)
- ATP activates PDH kinase (inhibits PDH)
order of reactions in the PDH complex
1) PDH w/ pyruvate
2) dHLT w/ 2-carbon molecule from pyruvate
3) dHLD w/ leftover lipoic acid
role of PDH in the PDH complex
cleaves pyruvate into CO2 and a 2-carbon molecule and then PDH's coenzyme (along with Mg 2+) thiamine pyrophosphate (TPP ; aka vitamin B1) forms a covalent bond with the 2-carbon molecule forming Acyl-TPP
- Rate-limiting step!!!
role of dHLT in the PDH complex (4 steps)
1) dHLT's coenzyme lipoic acid receives the 2-carbon molecule from Acyl-TPP and forms a covalent link
2) lipoic acid oxidizes the 2-carbon molecule creating the acetyl group now bonded via thioester linkage forming Acyl-lipoate
3) dHLT catalyzes an interaction between CoA and Acyl-lipoate causing the thioester bond to be broken and the acetyl transferred to CoA forming Acetyl-CoA
4) reduced lipoic acid interacts with dHLD to be reoxidized to repeat this process
role of dHLD in the PDH complex
dHLD's coenzyme FAD reoxidizes the reduced lipoic acid forming FADH2 which is later oxidized through an interaction with NAD+ yielding NADH, FAD, and H+
3 products from the PDH complex
Acetyl-CoA, NADH, CO2
*remember products double in catabolism of glucose*
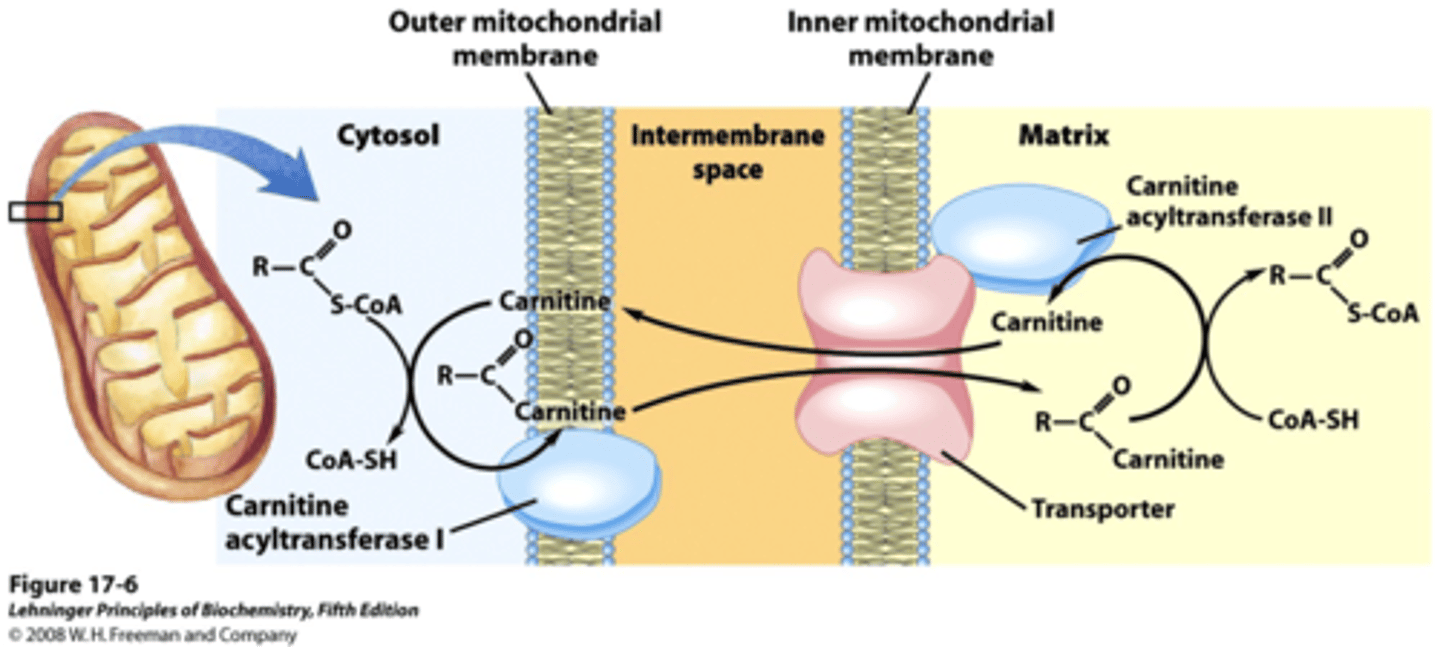
main function of the process to start beta-oxidation
use carnitine to shuttle an acyl group from CoA in the cytoplasm to CoA in the mitochondrial matrix
process of beta-oxidation
fatty acid enters the inter-mitochondrial space and bonds with CoA forming fatty acyl-CoA (FA-CoA), the fatty acyl group is transferred from the CoA to carnitine forming FA-carnitine to enter inner-mitochondrial membrane (FA-CoA cannot enter), FA-carnitine transfers FA group to mitochondrial CoA forming a mitochondrial FA-CoA and then beta-oxidation occurs forming Acetyl-CoA
how are ketone bodies associated with Acetyl-CoA?
Acetyl-CoA is used to produce ketone bodies when the PDH complex is inhibited but this reaction can also run in reverse causing ketone bodies to form Acetyl-CoA
how are amino acids used as ketone bodies?
amino acids lose amino group, get converted to ketone bodies which can form Acetyl-CoA
how does alcohol form Acetyl-CoA?
when alcohol is consumed in moderate amounts, alcohol dehydrogenase and acetaldehyde dehydrogenase can convert it to Acetyl-CoA
what is the problem with alcohol conversion to Acetyl-CoA?
when it is converted to Acetyl-CoA, NADH is also formed which accrues and inhibits the TCA cycle
- this version of Acetyl-CoA is used to synthesize fatty acids because of this
purpose of the TCA cycle
feed the electron transport chain (ETC) by loading up electron-shuttling molecules with electrons through reduction reactions
- all substrates and products are used over and over again with the exception of 4 them
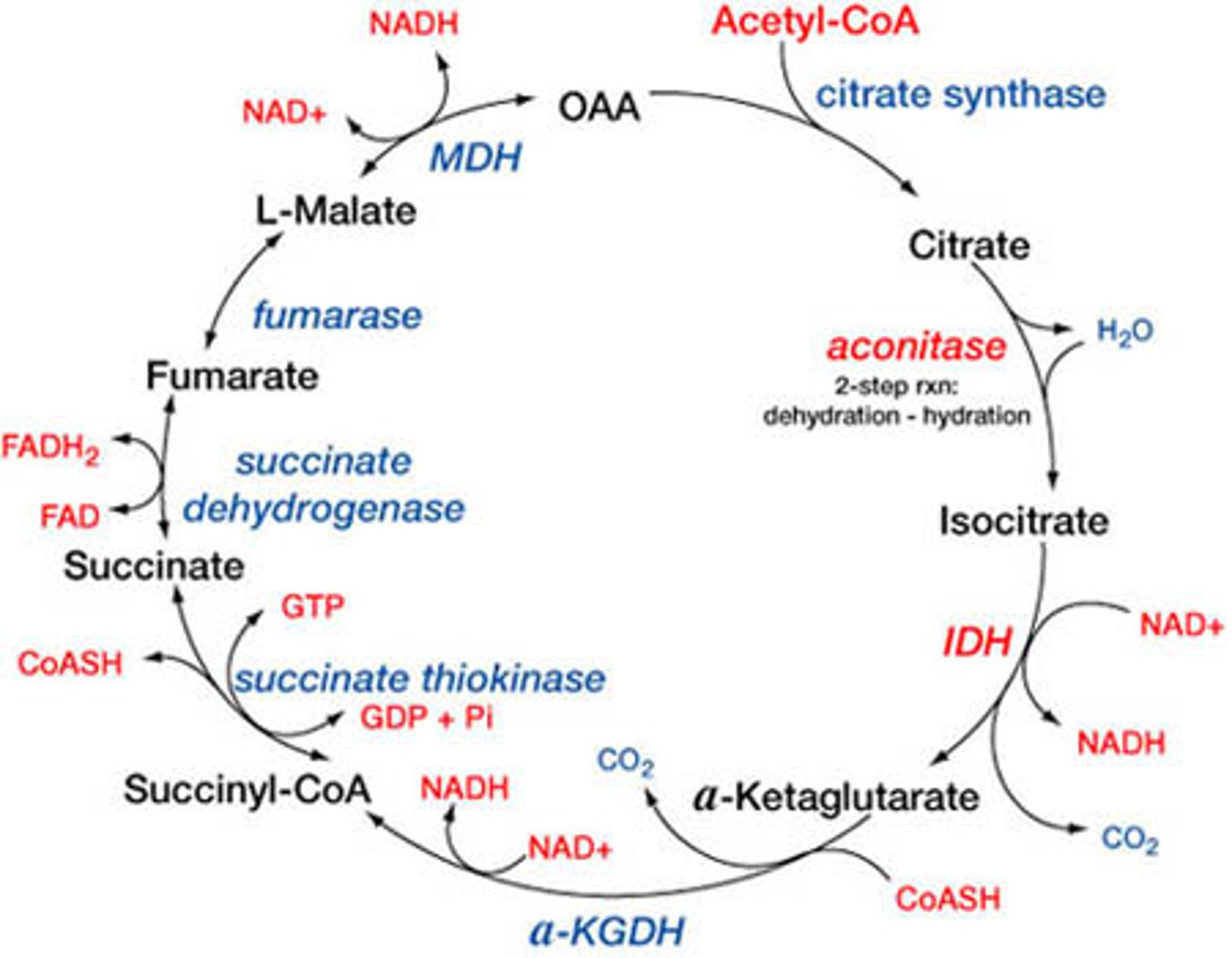
influence of oxygen on the TCA cycle
WILL NOT happen in anaerobic conditions even though oxygen is not directly used because the ETC DOES require oxygen and if there is none present then NADH and FADH2 will accumulate and inhibit the TCA cycle
acronym for every intermediate product in the TCA cycle
Please AC Can I Keep Selling Seashells For Money Officer?
- P,AC,C,I,K,S,S,F,M,O
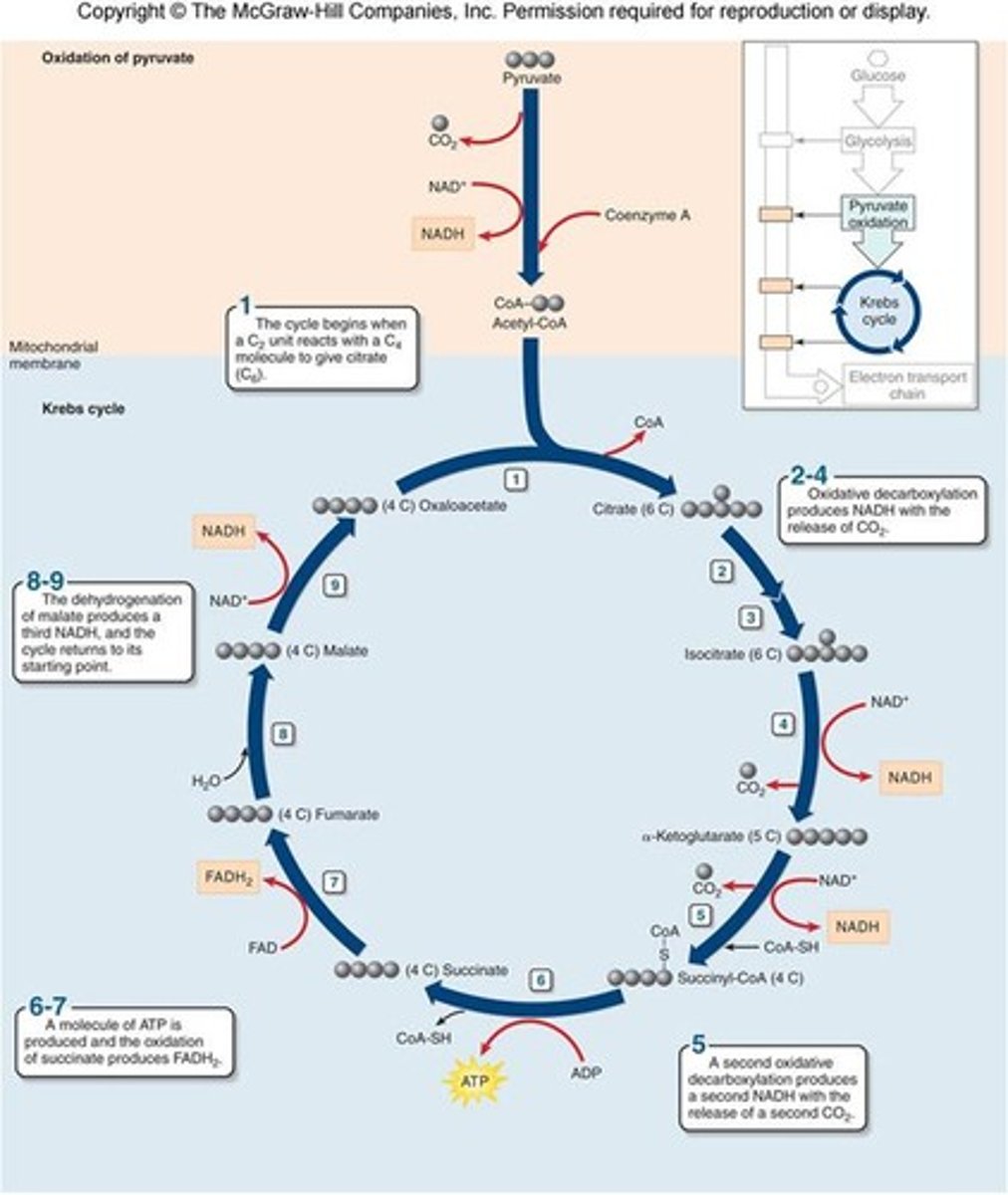
all ten intermediates in order starting with pyruvate
pyruvate, acetyl-CoA, citrate, isocitrate, alpha-ketoglutarate, succynil-CoA, succinate, fumarate, malate, oxaloacetate
8 steps in the TCA cycle
1) citrate formation
2) citrate isomerized to isocitrate
3) alpha-ketoglutarate and CO₂ formation
4) succinyl-CoA and CO₂ formation
5) succinate formation
6) fumarate formation
7) malate formation
8) oxaloacetate (OA) regeneration
3 NADH, 1 GTP, 1 FADH₂
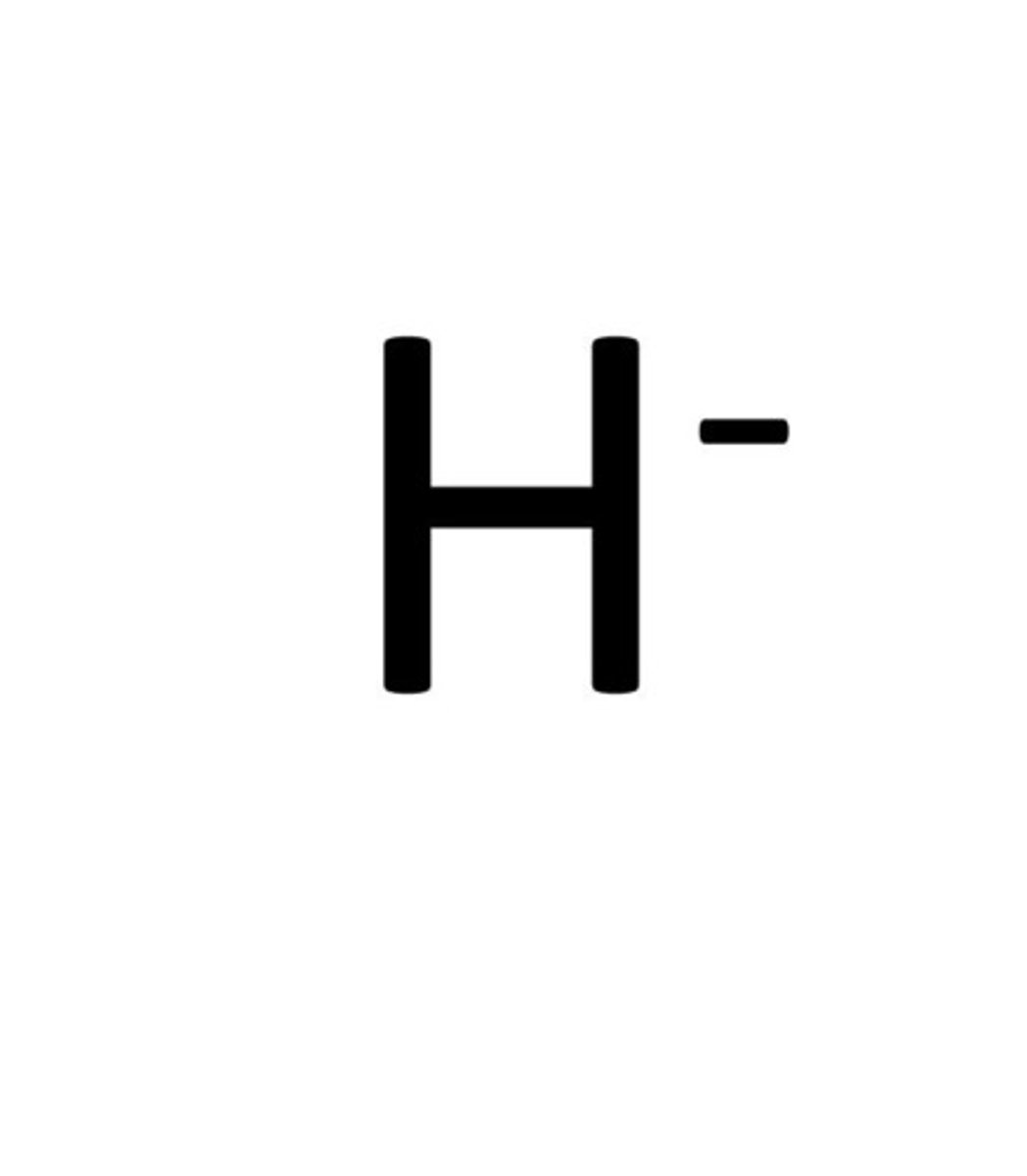
what are the roles of dehydrogenases in the TCA cycle?
transfer hydride ion to an electron acceptors like NAD+ and FAD
- are oxidoreductases
citrate formation
OA, Acetyl-CoA and water react to form citrate with help from citrate synthase
citrate isomerized to isocitrate
aconitase removes water and then adds water back changing stereochemistry making isocitrate
- aconitase is a metalloprotein and requires Fe 2+
alpha-ketoglutarate and CO2 formation (-1 carbon)
isocitrate forms alpha-ketoglutarate and CO2 with help from isocitrate dehydrogenase
- NADH is produced
importance of isocitrate dehydrogenase
rate-limiting enzyme for TCA cycle
succinyl-CoA and CO2 formation (-1 carbon)
alpha-ketoglutarate dehydrogenase complex (uses same conenzymes and cofactors as PDH complex) forms succinyl-CoA (contains thioester bond) and CO2
- NADH is produced
succinate formation
succinyl-CoA synthetase breaks thioester bond in succinyl-CoA forming succinate and CoA and releases energy to phosphorylate GDP making GTP (and then ATP)
- ONLY step in TCA cycle where ATP is directly produced
how does GTP help form ATP?
nucleosidediphosphate kinase transfers phosphate group from GTP to ADP making ATP
fumarate formation
succinate dehydrogenase turns succinate into fumarate
- ONLY step that occurs outside of mitochondrial matrix (occurs on/in the inner-mitochondrial membrane)
- FADH2 is produced
what is succinate dehydrogenase?
a flavoprotein
- means that is a protein that is bonded to FAD (or coenzyme like it)
why does fumarate formation occur outside of matrix?
succinate dehydrogenase (flavoprotein because it is covalently bonded to FAD, electron acceptor in this reaction) is an integral protein on the inner-mitochondrial membrane
why FAD and not NAD+ ?
succinate does not have enough reducing power to reduce NAD+
malate formation
fumarase converts fumarate to malate
when else is malate used?
in gluconeogenesis
- mitochondrial OA is converted to malate so it can leave the mitochondria and enter the cytoplasm which upon entering is converted back into OA
oxaloacetate regeneration
malate dehydrogenase converts malate to OA to continue cycle when there is more Acetyl-CoA present
- NADH is produced
total products from TCA cycle
1) 3 NADH
2) 1 FADH2
3) 2 CO2
4) 1 ATP (from GTP)
*remember products double in catabolism of glucose*
ATP production difference from FADH2 vs NADH in the ETC
FADH2 = 1.5 ATP
NADH = 2.5 ATP
total products and ATP production from PDH complex and TCA cycle
1) 4 NADH x 2.5 = 10 ATP
2) 1 FADH2 x 1.5 = 1.5 ATP
3) 1 GTP = 1 ATP
- So a total of 12.5 ATP are produced for every one pyruvate molecule (25 ATP for every glucose molecule)
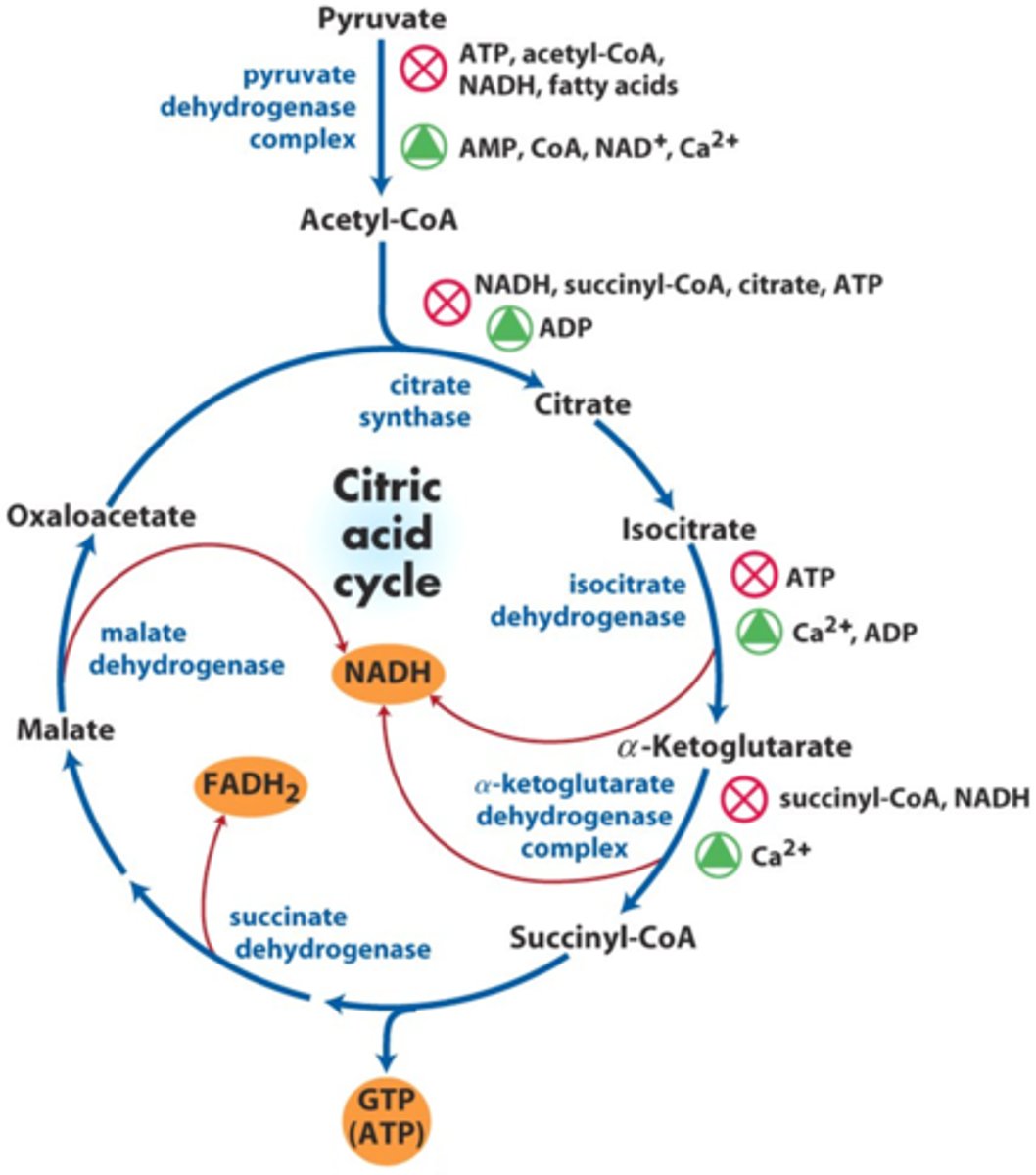
Regulation of the PDC complex and TCA cycle main theme
energy products inhibit the energy production processes
PDH complex regulation
- activated by ADP and enzyme pyruvate dehydrogenase phosphatase (in response to high leves of ADP)
- inhibited by ATP, NADH and Acetyl-CoA
3 TCA cycle regulation steps
1) citrate synthase (step 1)
2) isocitrate dehydrogenase (step 3)
3) alpha-ketoglutarate dehydrogenase complex (step 4)
why are these regulation steps?
these are the only three steps in TCA cycle that change the number of carbons from the reacting molecule
citrate synthase regulation
inhibited by ATP, NADH, citrate, succinyl-CoA
isocitrate dehydrogenase regulation
- activated by ADP and NAD+
- inhibited by ATP and NADH
alpha-ketoglutarate dehydrogenase complex regulation
- activated by ADP and Ca 2+
- inhibited by ATP, NADH, and succinyl-CoA
electron transport chain (ETC)
- integrated into the inner-mitochondrial membrane
- uses proton-motive force in order to facilitate the movement of electrons to oxygen which acts as a terminal electron acceptor (creates water)
general ETC mechanism
NADH and FADH2 give their electrons to ETC proteins which give the electrons to oxygen through a series of redox reactions
- while this is happening, protons are pumped into the intermembrane space to increase the proton gradient concentration
delta G for ATP formation
endergonic (positive delta G ; non-spontaneous)
delta G for the transport of electrons
exergonic (negative delta G ; spontaneous)
what is the function of the four complexes in the ETC?
facilitate and catalyze the electron transfer reactions
which three (of the four) complexes are sites where protons are pumped into the intermembrane space?
1) complex I (4 protons)
2) complex III (4 protons)
3) complex IV (2 protons)
what happens in complex I?
NADH transfers electrons to coenzyme Q (CoQ)
- complex I is made up of 20 subunits
2 most important subunits that help transfer electrons in complex I
1) protein with an iron-sulfur (Fe-S) cluster (in complexes I, II and III)
2) flavoprotein with coenzyme flavin mononucleotide (FMN) which is similar to FAD
(Oxidizes NADH)
important names for coenzyme Q seen throughout the ETC
- CoQ is ubiquinone
- CoQH2 is ubiquinol
movement of electrons within complex I (with the products of each reaction)
NADH to FMN (NAD+ and FMNH2) , FMNH2 to Fe-S cluster (FMN), Fe-S cluster CoQ (CoQH2)
- 4 protons pumped into intermembrane space
net results from complex I
NAD+ and CoQH2
what happens in complex II?
succinate transfers electrons to CoQ
three important subunits of complex II
FAD, succinate dehydrogenase, and Fe-S protein
movement of electrons within complex II (with the products of each)
succinate to FAD (fumarate and FADH2), FADH2 to Fe-S (FAD), Fe-S protein to CoQ (CoQH2)
- CoQH2 takes protons to complex III
- NO protons are pumped out
net results from complex II
fumarate and CoQH2
what happens in complex III?
CoQH2 (from complex II) transfers electrons to cytochrome c via the Q cycle
what is a cytochrome?
proteins with heme groups in which iron is reduced and then reoxidized (Fe 2+ to Fe 3+)
what is different about the transfer of electrons in complex III compared to the others?
cytochrome c can only carry one electron at a time so it must react with CoQH2 twice
movement of electrons within complex III (Q cycle)
- 2 electrons shuttled from CoQH2 to CoQ
- 2 electrons attached to heme are transferred to cytochrome c one at a time
- 4 protons pumped into intermembrane space
what happens in complex IV?
cytochrome c transfers electrons to oxygen which acts as the terminal electron acceptor
important enzyme in complex IV
cytochrome oxidase
- made up of cytochrome subunits a and a3
movement of electrons in complex IV
cytochrome oxidase transfers electrons from cytochrome c to oxygen forming water through a series of redox reactions
- Complex includes subunits of cytochrome a cytochrome a3 (together cytochrome oxidase) and Cu2+ ions
- 2 protons are pumped into the intermembrane space
effects of the proton-motive force (aka electrochemical gradient)
as proton concentration increases in the intermembrane space, the pH of the intermembrane space drops and the voltage difference between the intermembrane space and the matrix increases
- these two factors create the electrochemical gradient
important note about reduction potential
it increases along the ETC (I
what is the purpose of NADH shuttles?
to shuttle cytosolic NADH into the inner mitochondrial (via the use of another high-energy electron carrier)membrane because cytosolic NADH cannot cross by itself
what are the two NADH shuttles?
1) glycerol 3-phosphate shuttle
2) malate-aspartate shuttle
key players in the glycerol 3-phosphate shuttle (in order)
DHAP, cytoplasmic glycerol 3-phosphate dehydrogenase (G3PD) and NADH, glycerol 3-phosphate, G3PD-FAD,
what is G3PD-FAD?
an isoform of G3PD that is FAD dependent so it resides in the outer face of the inner mitochondrial membrane
how the glycerol 3-phosphate shuttle works
DHAP is converted to glycerol 3-phosphate by G3PD and NADH in the cytoplasm which then interacts with the FAD of the G3PD-FAD on the inner mitochondrial membrane creating FADH2 (which goes to the ETC) and DHAP
- makes 1.5 ATP per NADH molecule
key players in the malate-aspartate shuttle (in order)
1) cytoplasm: OA, malate dehydrogenase and NADH, malate
2) mitochondria: malate, malate dehydrogenase and NAD+, OA, aspartate transaminase, aspartate
how to malate-aspartate shuttle works
cytoplasmic OA is converted to malate by malate dehydrogenase and NADH which can cross into the mitochondrial matrix which then is converted to matrix OA by malate dehydrogenase and NAD+ creating NADH once in the matrix (which does to the ETC) ; OA can be converted to aspartate by aspartate transaminase which can go back to cytoplasm to be converted back into cytoplasmic OA
- makes 2.5 ATP per NADH molecule
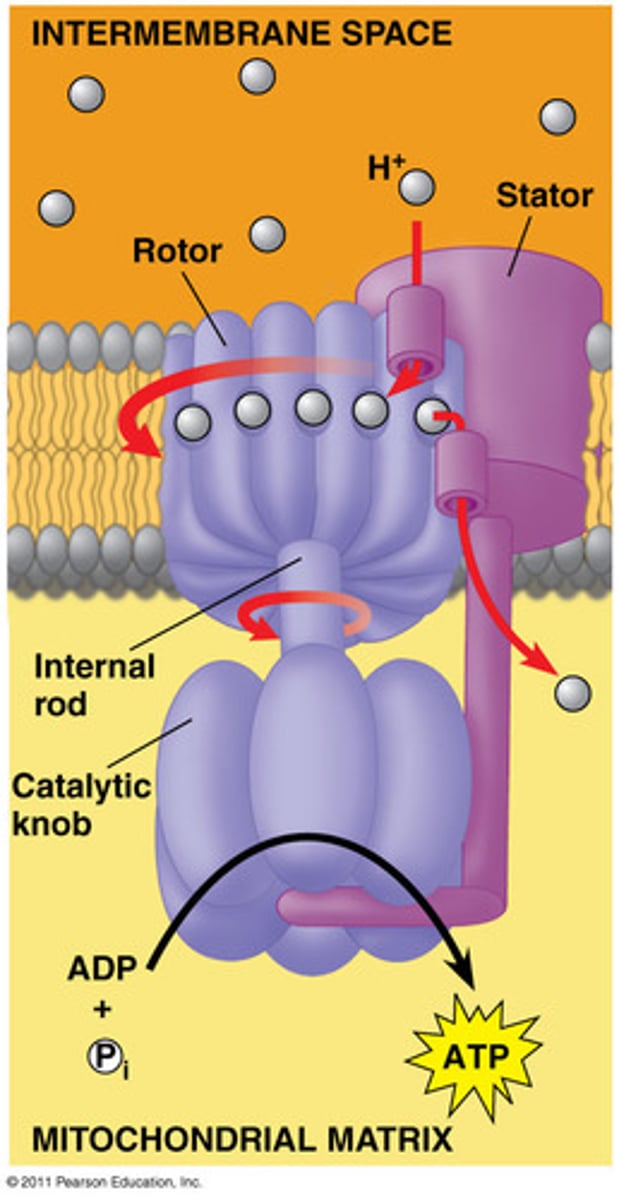
what is oxidative phosphorylation?
the coupling of ADP and Pi to make ATP by ATP synthase through the use of the electrochemical gradient established from the ETC
two parts of the ATP synthase
F0 and F1
role of the F0 part of ATP synthase
ion-channel that allows protons to move through it along gradient back into the matrix
role of the F1 part of ATP synthase
utilizes the energy from the protons flowing through F0 to phosphorylate ADP
2 possible mechanisms for this to occur
chemiosmotic coupling or conformational coupling
chemiosmotic coupling
ATP release is direct result of the electrochemical gradient where the ATP synthase remains stationary as protons flow through it
conformational coupling (accepted mechanism)
ATP release is an indirect result of the electrochemical gradient where the ATP synthase acts like a turbine which rotates to release ATP as a result of a conformational change due to the gradient
relationship between ATP synthesis and gradient
formation of ATP is highly exergonic and the dissipation of the gradient is highly endergonic
- these reactions can drive one another
name for the regulation of aerobic respiration
respiratory control
what is respiratory control?
the coordinated regulation of the TCA cycle, ETC and oxidative phosphorylation (ox-phos)
2 ways respiratory works:
1) if oxygen supply is ____ then ox-phos activity will decrease and NADH (and FADH2) levels will increase inhibiting the TCA cycle
2) if oxygen supply is ADEQUATE (or high) then ox-phos activity will become dependent on ADP and ATP ; if ADP levels are high and ATP levels are low then ADP activates _______ ________ which will increase the rate of the TCA cycle so the levels of NADH and FADH2 will increase causing an increased rate in the ETC and ATP synthesis and vice versa
LOW, isocitrate dehydrogenase
shortened version of respiratory control with low oxygen (number 1 from last card)
low ox-phos and high NADH and FADH2
- TCA cycle inhibited
shortened version of respiratory control with adequate oxygen (number 2 from last card)
high ADP => increased TCA cycle activity => increased NADH and FADH2 => increased ETC and ox-phos => more ATP