Ceramics
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
30 Terms
What types of bonding are present in ceramics?
Mostly ionic bonding, sometimes covalent
What elements make up ceramics?
Compounds of metallic and non-metallic elements
What is a cation in ceramics?
A positively charged metallic ion that has lost electrons
What is an anion in ceramics?
A negatively charged non-metallic ion that has gained electrons
What two factors dictate ceramic crystal structures
• Ion charge balance
• Relative ion sizes
What does Rc/Ra represent?
Ratio of cation radius to anion radius
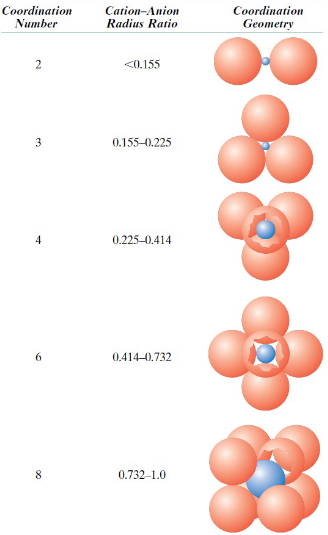
When is a ceramic structure stable?
When surrounding anions are all in contact with the cation
What is the coordination number?
Number of anions surrounding a cation
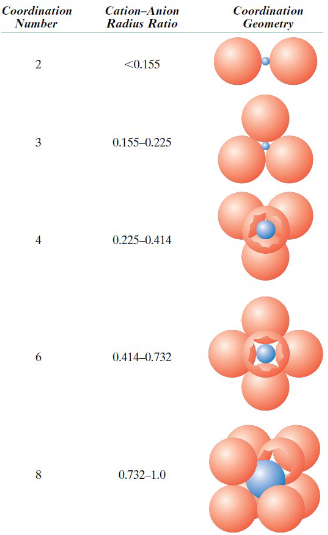
How is coordination number related to Rc/Ra?
Larger Rc/Ra = Higher coordination number
What is the basic unit of silicates?
SiO₄⁴⁻ anion (the silica tetrahedron)
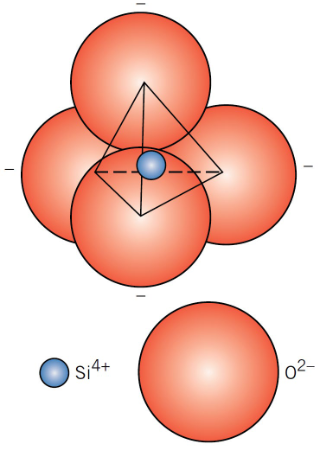
What do silicate properties depend on?
The packing of the silica tetrahedra and alloying additions of other metal oxides
Name the 3 crystalline polymorphs of silica
• Quartz
• Cristobalite
• Tridymite
How are layered silicates formed?
By sharing three oxygen atoms between tetrahedra
Why do layered silicates have a net negative charge?
Oxygen sharing leaves excess negative charge
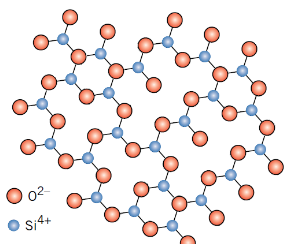
How does glass differ structurally from crystalline ceramics?
Glass is amorphous (non-crystalline)
Why is pure silica glass difficult to process?
Very high melting temperature (1200°C) and viscosity
What is the role of network modifiers in glass?
Break up the silica network while maintaining charge neutrality
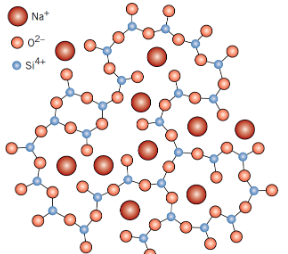
What effect do network modifiers have on the glass itself?
• Lowers the glass transition temperature (Tg)
• Softens the glass
What is Tg?
Glass transition temperature, where glass changes stiffness
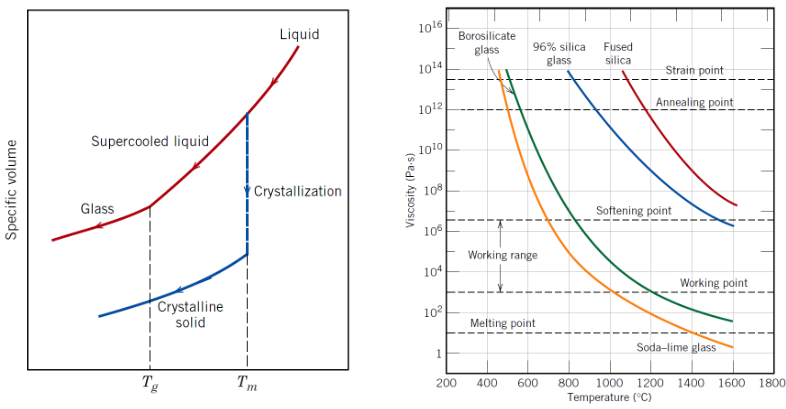
Do glasses have a sharp melting point?
No, they soften over a temperature range
Why are ceramics hard and wear resistant?
Strong ionic/covalent bonding limits dislocation motion
Why do ceramics resist corrosion and oxidation?
Many are already stable oxides
Why are ceramics strong in compression?
High shear strength due to dislocation motion being difficult

Why are ceramics brittle in tension?
They cannot plastically deform to relieve stress
Why is dislocation motion difficult in ionic ceramics?
Slip can force like charges together
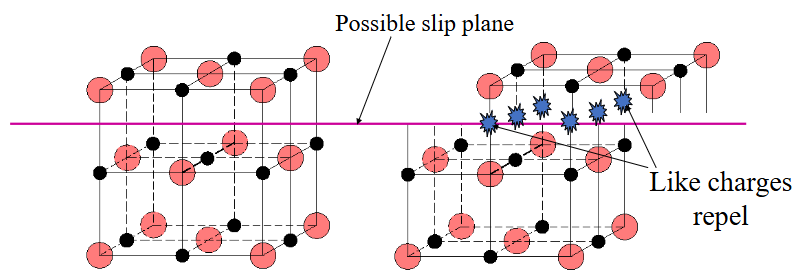
What is the result of having limited slip systems on a material?
High strength but extreme brittleness
What is concrete?
A ceramic composite (Gravel/sand in Cement matrix)
Why is steel used in concrete?
To provide tensile strength
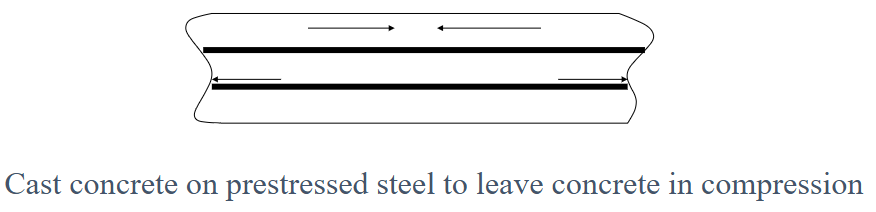
What is prestressed concrete?
Concrete in which internal stresses are introduced to counteract tensile stresses during use
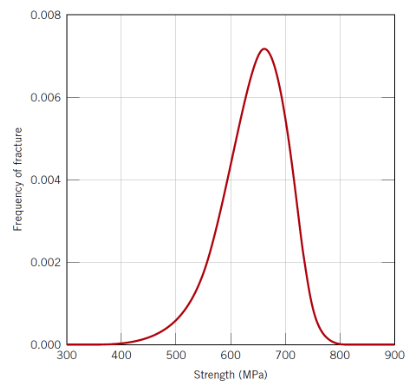
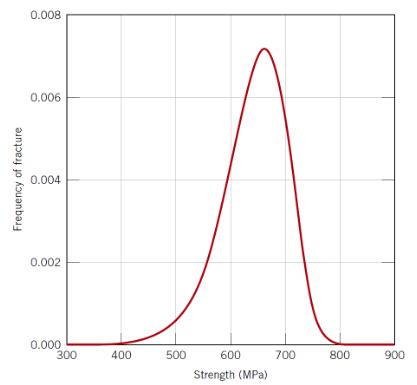
Why does ceramic strength show large scatter?
Variability in flaw size and location (i.e. the bigger the sample the more likely it is to contain a flaw and so the weaker it is)