Chemical Patterns and Reactions
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
50 Terms
Nucleus
The centre of an atom, which contains protons and neutrons, and accounts for the mass of the atom. The nucleus has an overall positive charge.
Protons
The number of protons determines the element.
positive charge,
have a mass
located in the nucleus.
Are attracted to electrons.
Known as subatomic particles
Neutrons
no charge (neutral)
located in the nucleus.
Known as subatomic particles
Electrons
negative charge
no mass (1840th of a proton and neutron)
orbit the nucleus (in shells/orbitals)
Are attracted to protons. The closer the electrons are to the nucleus, the greater the attraction to the protons will be.
Known as subatomic particles
Electrons in the same shell are the same radium from the nucleus.
Atom
The smallest particle of a substance, that can still be that substance. They compose all matter.
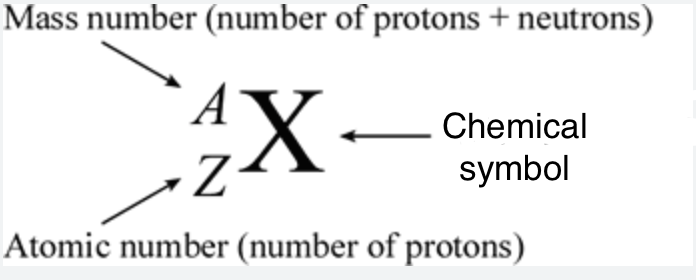
Atomic number
Equal to the number of protons
Mass number
Equal to the number of protons and neutrons in an element
Isotopes
Atoms with the same number of protons, but different number of neutrons, so atoms of the same element have different mass numbers.
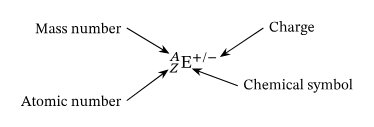
Ions
Atoms that have a positive or negative charge.
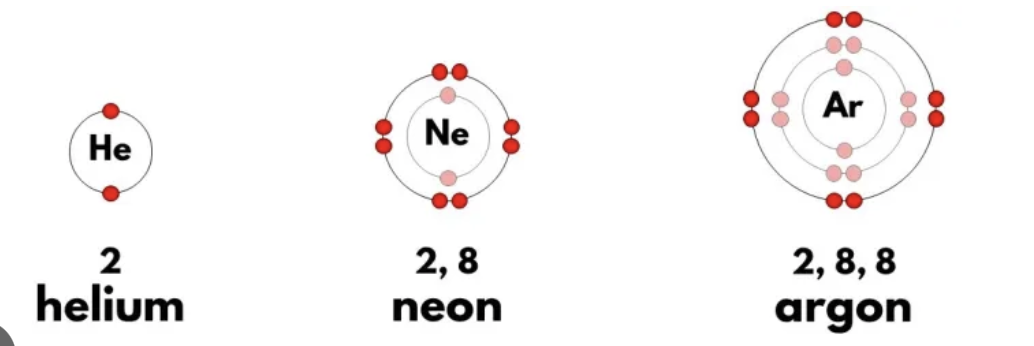
Valence electrons
Electrons in the valence shell (which can never have more then 8 electrons)
Valence electrons are the ones involved in chemical reactions.
Cations
A positively charged ion (it loses electrons)
Anions
A negatively charged ion (it gains electrons)
Octet Rule
Atoms prefer to have 8 electrons in their outer shell, to be as stable as possible
Periods
Horizontal rows on the periodic table.
Numbered 1-7
Elements in the same period have the same number of shells containing electrons.
eg. all atoms in period 4, have 4 shells
Groups
Vertical columns on the periodic table.
Numbered 1 - 18
Elements in the same group, have the same number of valence electrons in the outer shell, therefore they have similar chemical properties.
Alkali metals
Group 1 elements are alkali metals
They are highly unstable
Reactivity increases further down the columns
Have a +1 charge as ions
Alkaline earth metals
Group 2 elements are alkaline earth metals
They are highly unstable
Reactivity increases further down the columns
Have a +2 charge as ions
Halogens
Group 17 elements are halogens
Unstable
Reactivity increases higher up the column
Have a -1 charge as ions
Noble gases
Group 18 elements
Stable (full valence shell)
Highly un reactive (full valence shell)
Exist as single atoms
Atomic radius
The distance from an atom’s nucleus to the valence shell
The smaller the atomic radius, the stronger the attraction, and the higher the reactivity
Reactivity
A substances tendency to chemically react with another substance
Metals
Solid at room temperature (except for mercury)
Conducts heat and electricity proficiently
Generally hard but brittle
Malleable and ductile
Lustrous
High melting point
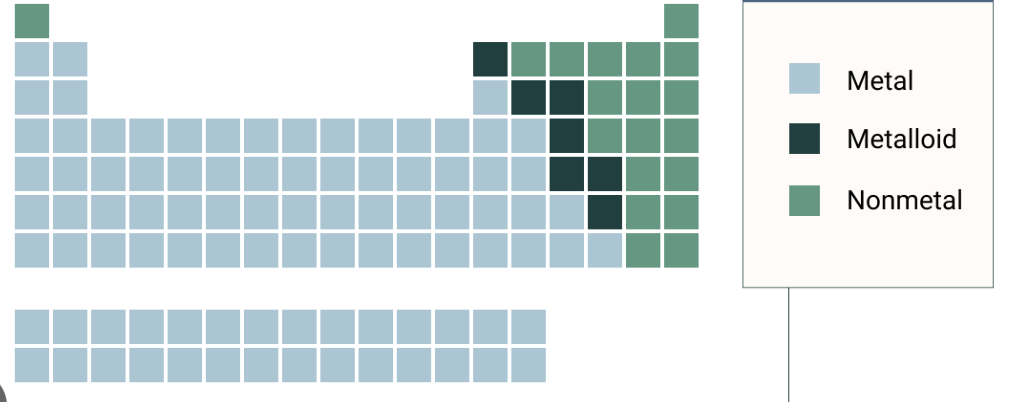
Non-metals
Essentially the opposite of metals
Poor conductors of heat and electricity
Dull, brittle, unmalleable
Low melting point (generally gases at room temperature)
Metalloids
Behave like metals, and non-metals (semi-conductors)
Transition metals
Similar properties to main group metals
Groups 3-12
Ionic bonding
Occurs between a metal and non-metal
The atom will lose or gain electrons with another atom, so a cation and anion are formed
The metal becomes stable by giving away its valence electrons (cation +)
The non-metal gains the electrons the metal loses (anion -)
Both the atoms will now have full, and therefore stable valence shells
This cation and anion have opposite charges to one another (eg 2+, 2-) so the net charge will be 0
These opposite charges (cations and anions) are strongly attracted to one another, which bonds the atoms
No electrons are lost in ionic bonding, rather they are just transferred.
Molecules
Two or more bonded atoms.
Lattice
The continuous arrangement of bonded atoms in regular patterns.
Ionic lattices are held by electrostatic attraction, and they form hard, rigid, and brittle substances (crystals).
Ionic substances
Do not conduct electricity as solids
Conduct electricity in molten states
High melting point
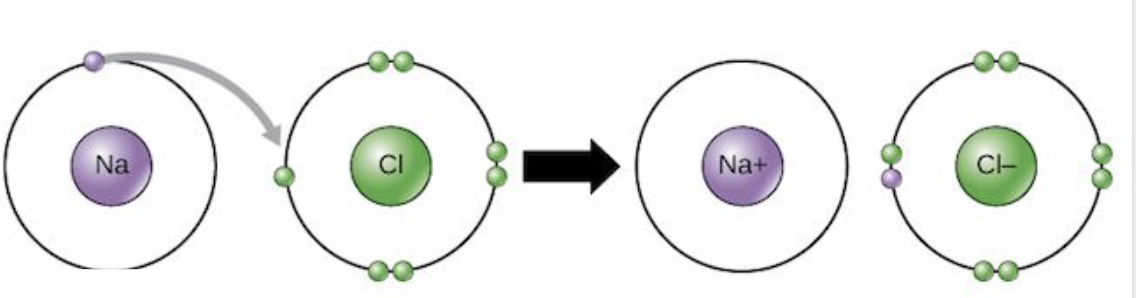
Electron transfer diagram
A diagram that represents the transfer of electrons during ionic bonding between atoms.
include electrical configuration
include charges (for ions)
include arrows to show the transfer of electrons
Ionic formula
Ensure that the net charge is 0
Do NOT write the charges in the formula
If the amount of a molecule is being changed, then the molecule must be put in brackets to ensure that the subscript applies to the entirety of it
The name of the metal does not change
The name of the non-metal does change
If it is a singular element and ‘IDE’ is added (eg. chlorine become chloride)
If the non-metal is a compound itself, then the name does not change (eg. sulfate, carbonate)
Metallic bonding
Occurs between a metal and a metal
Metals become cations
Metals have the structure of being a layer of cations surrounded by a sea of delocalised electrons
The cations are arranged in a closely packed structure
While the delocalised electrons are free to move throughout the lattice, belonging to the lattice as a whole.
They are held in the lattice through electrostatic forces of attraction
The net charge is 0
Metallic substances
Have the properties of metals
These properties are explained through metallic bonding
Electricity conductor: the delocalised electrons carry the current from negative to positive
Heat conductor: the electrons vibrate to transfer energy, and when heated, they vibrate more
Lustrous: the delocalised electrons reflect heat
Malleable and ductile: the layer of cations can be forced across each other
High melting and boiling points: Large amounts of heat energy must be applied to break the electrostatic attraction
Dense: closely packed structure
Covalent bonding
Occur between a non-metal and non-metal
The octet rule is fulfilled by atoms sharing their valence electrons with one another.
These shared electrons become the “glue“ that keeps the atoms bonded
One of the strongest bonds in chemistry
Certain non-metals always exist as a pair of atoms (eg.H2, O2, F2)
Covalent substances
Low melting and boiling point: because the bond has to broken, rather then intermolecular forces
Are liquids or gases at room temperature
Do not conduct electricity
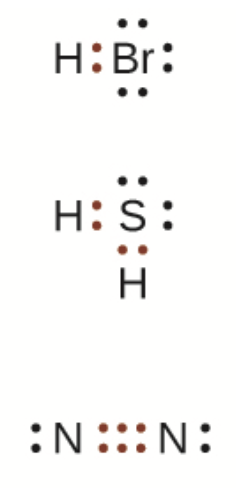
Lewis diagram/electron dot diagram
Paired electrons are referred to as “lone pairs” (or non-bonding electrons)
Bonded electrons are referred to as “bonded electrons”
Used to covalent molecules
The atom with the most bonding electrons is placed in the middle
Except for hydrogen, all atoms must have 8 electrons around them
All electrons will share as many electrons as they need to gain
Valence structures
Another representative of covalent molecules
Each shared pair of electrons is replaced by a dash
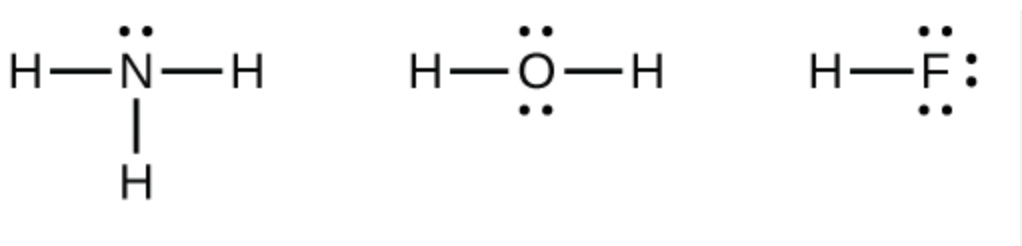
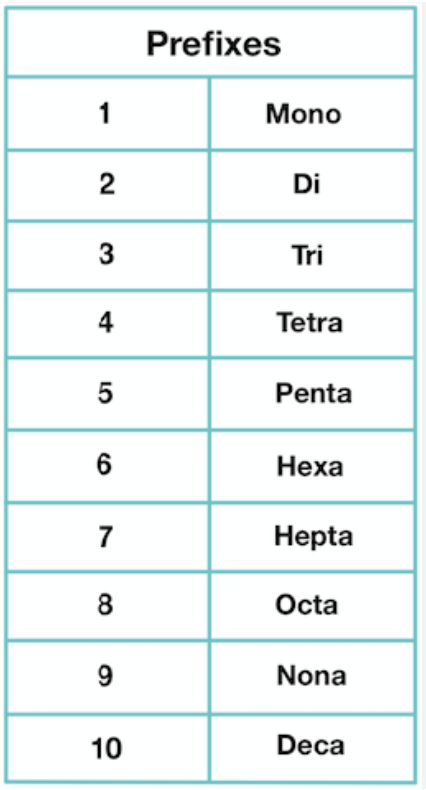
Forumla names
Prefixes are applied to convey information about the number of atoms in each molecule of a non metal’s name.
State symbols
solid (s)
liquid (l)
aqueous (aq)
gas (g)
Chemical reactions
Reaction during which atoms are rearranged, so that the products are not the same as the reactants
Chemical change must occur, in the formation of a new chemical product
Reactants
Chemicals pre reaction
Products
Chemicals post reaction
Law of Conversation of Mass
Hinges on the principle that matter cannot be destroyed or created, only rearranged
So during a chemical reaction, atoms are just rearranged
Therefore mass cannot be created or destroyed
So the mass of the reactants will be equal to the mass of the products
Law of Constant Proportions
Some chemicals will always exist in certain proportions (rations)
Chemical equations
Are balanced when the reactants are equal to the products
Chemical equations are balanced when the Law of Conversation of Mass is satisfied
Equations can only be balanced by changing the coefficient
The subscript can never be changed
reactants > products
Write the word equation
Write the chemical formula of each substance (include states)
Balance the equation
Combination reactions
AKA synthesis reaction
Two chemical reactions form one product
A + B > AB
Decomposition reactions
One reactant forms several products
AB > A + B
Precipitation reactions
A and C, two cations react with B and D, two anions
These two aqueous substances produce an aqueous substance, and a precipitate
AB (aq) + CD (aq) > CB (aq) + AD (s)
Precipitate
An insoluble, solid compound
Solubility
Whether a substance can be dissolved into water
Determined via a solubility table