Organic Chem 261 - Exam 1
1/28
Earn XP
Description and Tags
Chapter 1, Chapter 2, Chapter 3 = naming alkanes, carbon numbers, Newman Projections
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
29 Terms
Methane
1 carbon
Ethane
2 carbon chain
Propane
3 carbon chain
Butane
4 carbon chain
Pentane
5 carbon chain
Hexane
6 carbon chain
Heptane
7 carbon chain
Octane
8 carbon chain
Nonane
9 carbon chain
Decane
10 carbon chain
Docane
12 carbon chain
sp3 hybridized carbon
109.5 angle, tetrahedral, 4 sigma bonds
Sp2 hybridized carbon
3 sigma bonds 1 pi bond,120, trigonal planar
Sp hybridized carbon
180 angle, Linear, at least 2 pi bonds
Carbon bonding patterns
4 bonds
Hydrogen bonding patterns
1 bond
Halogen bonding patterns
1 bond, 3 lone pairs
Oxygen bonding patterns
2 bonds, 2 lone pairs
Nitrogen Bonding patterns
3 bonds, 1 lone pair
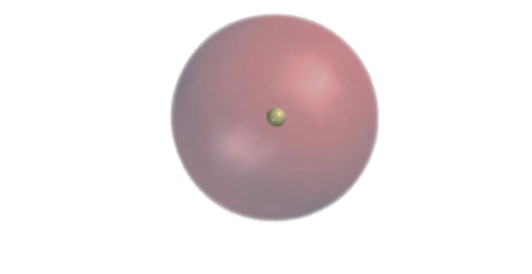
what orbital is this?
A s orbital
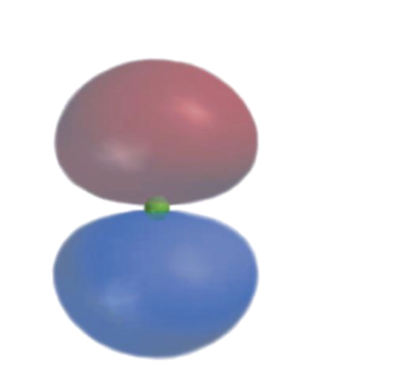
what orbital is this?
A p orbital

what orbital is this?
sp3
Boron bonding patterns
3 bonds (incomplete octet)
Sp3 Character
25%s, 75%p
Sp2 Character
33.33% -s, 66.66%- p
Sp Character
50/50
Electronegativity trend
up and to the right
Atomic size
down and to the left
Higher electronegativity difference equals
More polar