POC LAB - Qualitative Analysis of Elements in Organic Compounds
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
Carbon
Element with an atomic weight of 6
Hydrogen
Element with an atomic weight of 1
Oxygen
Element with an atomic weight of 8
Nitrogen
Element with an atomic weight of 7
Sulfur
Element with an atomic weight of 16
Phosphorus
Element with an atomic weight of 15
Silicon
Element with an atomic weight of 14
Flourine
Element with an atomic weight of 9
Chlorine
Element with an atomic weight of 17
Bromine
Element with an atomic weight of 35
Iodine
Element with an atomic weight of 53
Qualitative Analysis
Systematic approach in identifying elements in organic compounds using qualitative chemical reagents which heavily relies on organoleptic approach
Cupric Oxide
upon heating this substance, carbon and hydrogen oxidized to carbon dioxide and water
Lime water
used to detect presence of CO2 after heating Cupric Oxide
Anhydrous cupric sulfate
used to detect presence of water after heating Cupric Oxide
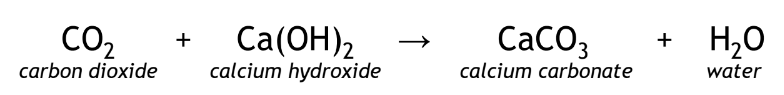
clear solution becomes turbid
Positive result of CO2 reacts with Ca(OH)2 forming CaCO3 and H2O
white powder turns to blue
Positive result of H2O reacts with anhydrous CuSO4 forming CuSO4 • 5 H2O
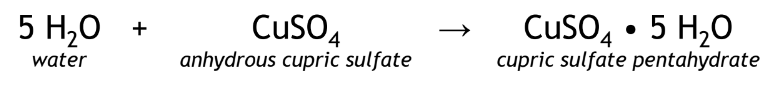
Nitrogen, Halogens, and Sulfur
Organic compounds that do not ionize and are covalent bonds
Sodium Metal
Compound fused in covalent organic compounds to convert into inorganic compounds
Lassaigne’s Extract
alkaline solution obtained by extracting the fused mass in water
Sodium Cyanide (NaCN)
This reaction creates what product?

Sodium Halide (NaX)
This reaction creates what product?

Sodium Sulfide (Na2S)
This reaction creates what product?
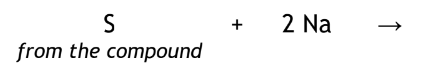
Blue
The litmus paper must turn this color after being moistened with ammonia gas from heating amino nitrogen
Pungent Odor
Positive result of Soda Lime Test
Beilstein Test
it is a flame test used to detect presence of halogens
Green Colored Flame
Positive result of Beilstein Test
Negative Result
result of fluorine under beilstein test
Positive Result
indicates presence of halogens/organic compounds based on the test
Silver Nitrate Test
the halogen reacts with silver nitrate in the presence of dilute nitric acid forming insoluble silver halide
White to light yellow precipitate
Positive result of presence of silver halide under silver nitrate test
Lead Acetate Test
test for identifying presence of sulfur
brownish-black precipitate
Positive result of sulfur upon reaction with lead acetate
Cysteine
amino acid found in Albumin
PbS
reacts with cysteine forming a black precipitate
Heating
causes the breaking of the polypeptide chain
Ferrox Test
Test that uses iron (III) hexathiocyanatoferrate or ——- paper to identify the presence of oxygen
red to reddish-purple color
Positive result of the ferrox test
ferrox paper
prepared by soaking a filter paper in methanol with equal parts of ferric chloride and ammonium thiocyanate
deep red colored solution
complex FeSCN2+ yields what color