Receptors 4
1/22
Earn XP
Description and Tags
Drug-Receptor interactions
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
Lock and key model
the drug is complementary to the receptor
induced fit model
both the drug and the receptor both adjust their shapes to provide an optimal fit
conformational-selection model
protein receptor fluctuates between several conformational states. only some of these states allow binding to the drug/ligand
Drug targets include
proteins (receptors, enzymes, binding sites on ion channels)
nonproteins (DNA)
Pharmacophore
minimum chemical features necessary to elicit the biological response at a given biological target
Pharmacodynamics
the study of the effects of drugs on the body. this includes the interaction of drugs with their biological targets
covalent bonds
irreversible link to the receptor
50-150 kcal/mol
seldom formed in drug-receptor interaction (exceptions: drug + enzyme or drug + DNA)
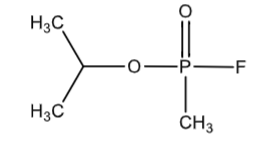
Sarin
nerve gas - organophosphorus compound
covalently bonds to AchE on the Ser residue
Ionic bonding
results from the attraction of oppositely charged groups on the drug and receptor
5-10 kcal/mol
strongest of noncovalent interactions
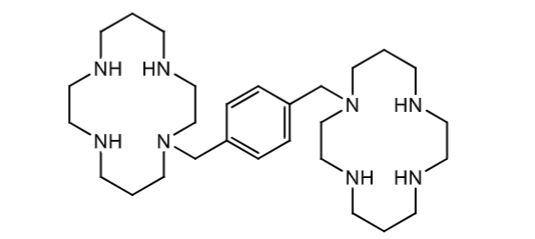
Plerixafor
competitive antagonist of CXCR4 receptor on leukocytes
amine groups form ionic bonds with acidic amino acids
dipole
when a carbon is bound to an electronegative atom, there is an asymmetric distribution of electrons in the covalent bond. The electrons lie closer to the electronegative atom.
This gives the carbon a partial positive charge and the electronegative atom a partial negative charge
Ion-dipole or dipole-dipole interactions
attractive forces between permanent dipoles on polar groups within a drug molecule and receptor (or an ion and a dipole)
1-7 kcal/mol
proper alignment is required for these interactions to occur
Relative strength of electrostatic interactions
ionic bond > ion dipole > dipole dipole
Hydrogen bonding
a type of dipole-dipole interaction
formed between a proton of group X-H (X = electronegative) and another electronegative atom which contains an unshared pair of electrons
2-5 kcal/mol
H-bond acceptors
ketone
ester
ether
thioether
disubstituted amides
disubstituted carbamates
pyridines
fluorine
Hydrogen bond donors
pyrrole
protonated amine
Hydrogen bond donors + acceptors
alcohol or hydroxyl
phenol
amide
unionized primary or secondary amines
unionized carboxylic acid
carbamate
urea
thiol
Cation-pi interactions
interaction between a cation and the center face of an aromatic ring
2-4 kcal/mol
aromatic rings in Phe, Trp, Tyr may interact with a positively charged functional group on a drug
positively charged side chain of Arg or Lys may interact with an aromatic ring on a drug structure
salt bridge
noncovalent interaction between 2 ionized groups. mix of a hydrogen bond and an ionic interaction
pi-pi interactions
interaction between aryl rings
2 styles
t-shaped edge to face
parallel displaced stacking
hydrophobic interactions
describes the tendency of nonpolar compounds to transfer from water to an organic phase
attraction between nonpolar groups on a drug and lipophilic regions on a receptor
widespread because nearly all drugs have nonpolar parts
0.7 kcal/mol
Van der Waals Attractions
an attraction between nonpolar portions of 2 molecules due to a temporary dipole in a C - C covalent bond within one molecule, which induces an opposite dipole in the approaching molecule
0.5 kcal/mol
nonpolar surface area determines the force of the attraction
optimal drug-receptor interactions
3-5 points of contact
many weak interactions = strong collective interaction