1.2 Draw diagrams/pictures of the various models of the atom as they changed over time.
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
1
New cards
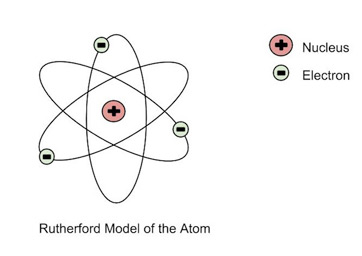
Rutherford’s Model (1911) - “Nuclear Model”
Rutherford's experiment showed that atoms have a dense, positively charged nucleus at the center, with electrons orbiting around it.
2
New cards
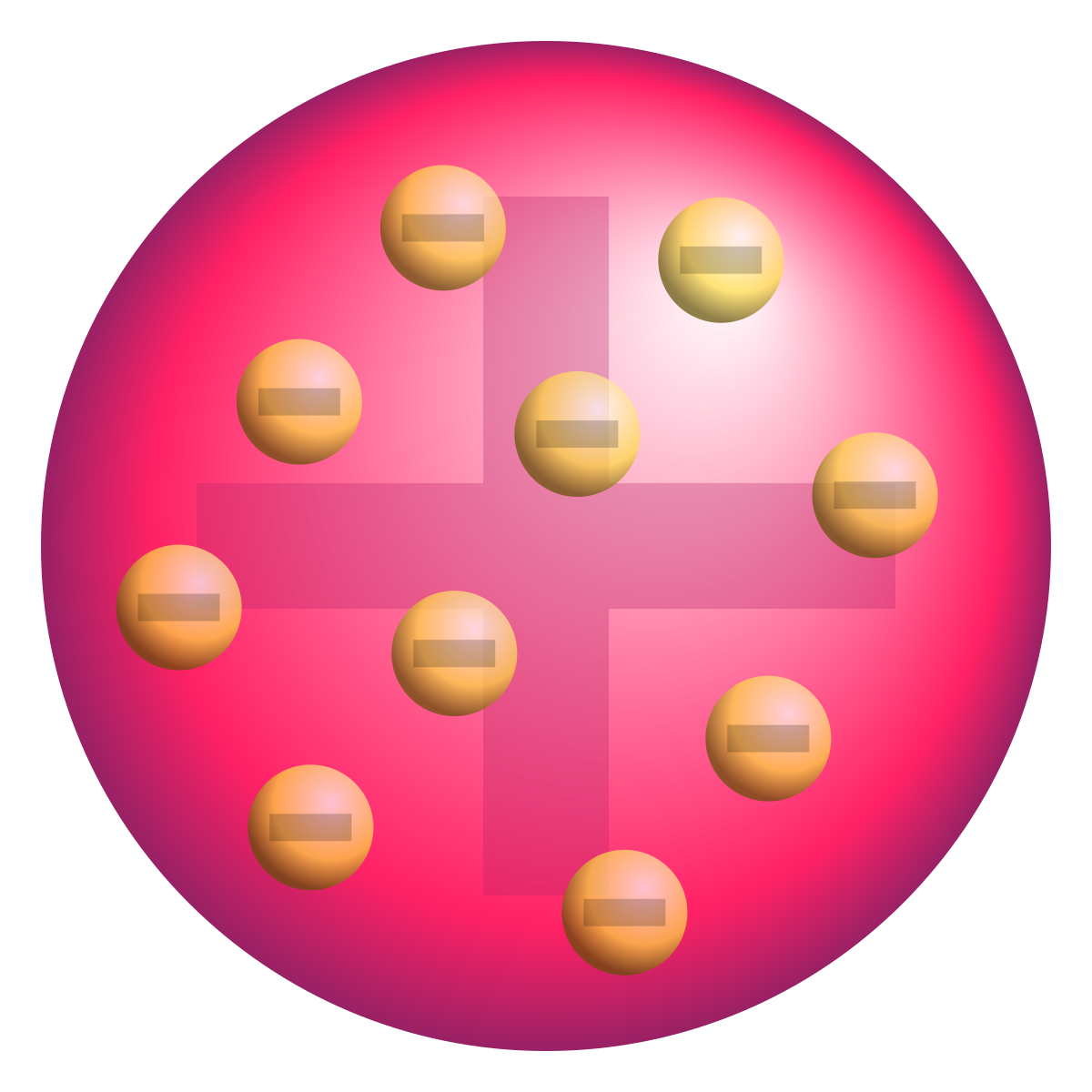
Thomson’s Model (1897) - “Plum Pudding Model”
Thomson proposed that atoms were made of a positively charged "pudding" with negatively charged electrons embedded inside, like raisins in a pudding.
3
New cards
Dalton’s Model (1803) - “Billiard Ball Model”
Dalton believed atoms were tiny, indivisible spheres that combined to form compounds in fixed ratios.
Picture: Inside the model, there were atoms combined in fixed ratios (for example, two circles together for H₂, three circles together for H₂O).
4
New cards
.
.