Physical Chemistry
1/109
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
110 Terms
ΔHat⦵
is the enthalpy change when 1 mole of gaseous atoms is formed from its elements under 298K and 1atm
Mg(s) → Mg(g)
ΔHat⦵ type of energy change and reason
Always Endothermic; energy is required to break down bonds between elements
ΔHlatt⦵
enthalpy change when 1 mole of ionic compound is formed from its ions in the gaseous state under 298K and 1atm
X-(g) + Y-(g) → X+Y-(s)
ΔHlatt⦵ type of energy change and reason
Always Exothermic; energy is released when new bonds are made.
(more exothermic = stronger ionic bond)
ΔHEa1⦵
is the enthalpy change when 1 mole of electrons is added to 1 mole of gaseous atoms to form 1 mole of gaseous 1- ions under 298K and 1 atm
Cl(g) + e- → Cl-(g)
ΔH Ea type of energy change and reason
Ea1 :
Exothermic; energy is released when new bonds are made
Ea2 onwards :
Endothermic; energy is required to overcome repulsion between electrons and negative ions
Factors affecting the magnitude of electron affinity
Nuclear charge :
Nuclear charge ↑ = attractive forces between electron and nucleus ↑
Distance :
Distance between nucleus and outermost shell ↑ = force of attraction ↓
Shielding effect of electron :
Number of shells ↑ = Shielding effect ↑ = force of attraction ↓
Does Cl have a more exothermic EA1 than S? and why?
Yes :
Positive nuclear charge ↑
Shielding effect =
Distance ↓
Attractive forces between nucleus and incoming electron ↑
therefore, Cl is more exothermic
Trend of EA in group 16 and 17
Becomes less exothermic :
Positive nuclear charge ↑
Shielding effect ↑ and increase in distance outweighs ↑ in nuclear charge
Forces of attraction between nucleus and incoming electron ↓
so its magnitude decreases
Exceptions to the group 16 and 17 EA trend
Fluorine :
Very small atomic radius
Electron density is high
More repulsion between incoming electrons and electrons already present at fluorine
Less attractive force between nucleus and incoming electron
It is less exothermic than expected
Order for born haber cycle
ΔHf (start cycle)
ΔHlatt (end cycle)
ΔHat (metal)
IE (metal)
ΔHat (non-metal)
EA1
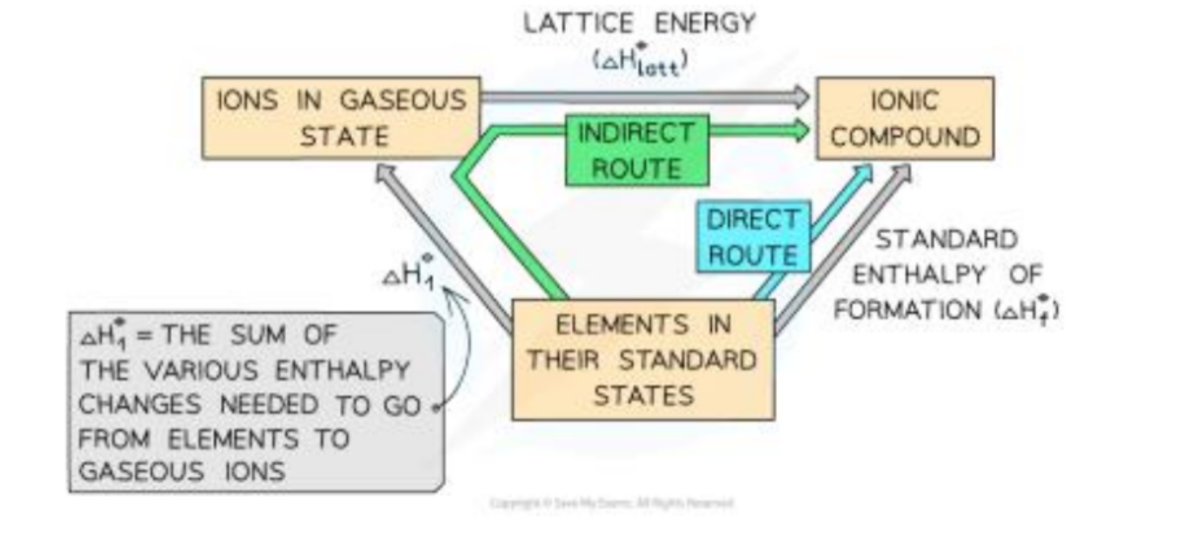
First Ionization energy
energy needed to remove one electron from each atom in one mole of atoms of the element in the gaseous state to form one mole of gaseous ions.
M(g) →M+(g) +e-
ΔHf⦵
is the enthalpy change when one mole of a compound is formed from its elements under 298K and 1atm
Na(s) + (1/2)Cl2 → NaCl(s)
Factors affecting ΔHlatt⦵ and how
Size of the ions
when the size of the ion ↑:
-the charge density (charge/size) of the ion will ↓.
-forces of attraction between the ions ↓.
-lattice energy becomes less exothermicCharge on the ions
when the charge of the ion ↑:
-the charge density of the ion will ↑
-forces of attraction between the ions ↑
-lattice energy becomes more exothermic
(Note : After stating if size/charge is the one changing mention change in charge density aswell)
Na+ has similar size with Ca2+, why is the lattice energy of NaCl less exothermic than CaCl2
this is because CaCl2 has a greater charge since Ca2+ has a greater charge density than Na+ which means forces of attraction between ions are greater, more energy will be released when the new bonds are formed between them.
Polarising power
Ability of the cation to attract electrons and hence distorts an anion.
The more polarised an ionic bond is, the greater covalent character, so an ionic bond is perfect if there is complete separation of charge
Factors affecting ion polarisation
Charge density of cation:
Greater charge density means the polarising power of the cation is higherSize and charge of anion:
Greater size of anion means higher polarisability
Charge of anion becoming more negative means higher polarisability
Trend of thermal stability of group 2 carbonates and nitrates
Since :
Atomic number increases
Cation size increases, charge remains constant so charge density decreases
Polarising power of the cation decreases down the group. (stronger ionic bonds)
Thermal stability will increase down the group
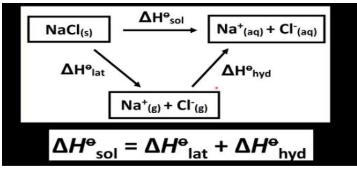
ΔHsol⦵
Energy when 1 mole of ionic solid dissolves in very sufficient amount of water to form a very dilute solution under 298K and 1atm
NaCl (s) + aq → Na+(aq) +Cl-(aq) [ which is just NaCl(aq) ]
ΔHsol⦵ type of enthalpy
can be exothermic and endothermic ;
the more exothermic it is, the more likely it is to be soluble in water
(if its a positive value; then entropy is considered)
ΔHhyd⦵
the enthalpy change when 1 mole of a gaseous ion dissolves in sufficient water to form a very dilute solution under 298K and 1atm.
Ca2+(g) + aq → Ca2+(aq)
ΔHhyd⦵ type of enthalpy change
always exothermic because when an ion dissolves in water; new bonds are formed between ions and water molecules
ΔHhyd⦵ factors
Size
Size increases = less exothermic
Charge
Charge increases = more exothermic
Solubility trends for group 2 sulfates
Solubility depends on the balance between ΔHlatt⦵ and ΔHhyd⦵
ΔHlatt⦵ and ΔHhyd⦵ both become less exothermic due to increase in cation size and decrease in charge density.
ΔHhyd⦵ will become less exothermic faster due to SO4 large size
Enthalpy change in solution will therefore become less exothermic
Solubility will decrease down the group
Solubility trends for group 2 hydroxides
Solubility depends on the balance between ΔHlatt⦵ and ΔHhyd⦵
ΔHlatt⦵ and ΔHhyd⦵ both become less exothermic due to increase in cation size and decrease in charge density.
ΔHlatt⦵ magnitude will decrease exothermic faster due to OH size
Enthalpy change in solution will therefore become more exothermic
Solubility will increase down the group
What does solubility depend on?
The balance between ΔHlatt⦵ and ΔHhyd⦵
Calculation of ΔHsol⦵ from lattice energy and Enthalpy change of hydration
correction : its -delta hlatt

Entropy and its unit
measure of the degree of disorder of a system
unit = Jmol-1k-1
A system is more stable when its energy is more spread out in a more disordered state.
Factors that contribute to increase in entropy
Solids melting
Temperature increase
Liquids boiling
Increase in number of gas molecules
ionic solids dissolve in water
CaCO3 has a greater entropy than CaO despite being in the same state,explain
more atoms present in CaCO3 (5) than CaO (2)
More disorder in CaCO3
Spontaneous/Feasible Reaction
a reaction that will occur naturally without external influence
how to calculate ∆Ssys
∆Ssys= ΣSproducts-ΣSreactants
∆G equation and units for each
∆G=∆Hreaction-T∆Ssys :
(∆G<0;spontaneous)
∆G=kJmol-1
∆Hr = kJmol-1
T = K
∆Ssys= Jk-1mol-1 (conversion to kJ → divide 1000)
minimum temperature where reaction is feasible (∆G=0)
0K (or -273.15 C → convert!!)
feasibility of a reaction can be altered by
Temperature change
Generally, exothermic reactions are (Spontaneous/Non-Spontaneous) and why?
Exothermic reactions are spontaneous in general :
energy is released to the surroundings increasing its arrangements.
which causes increase in entropy
Generally, endothermic reactions are (Spontaneous/Non-Spontaneous) and why?
Endothermic reactions are non-spontaneous in general :
Energy is absorbed from the surrounding, decreasing its arrangements
which causes decrease in entropy
(Inorganic Part) decomposition of group 2 nitrates - Magnesium nitrate eg
2Mg(NO3)2 (s)→2MgO(s) + 4NO2(g) + O2(g)
standard conditions
pressure of 1atm (or 101kPa)
temperature 298K
ions at a concentration of 1.0moldm-3
Standard electrode potential (E0)
emf of cell when a half-cell is connected to a standard hydrogen electrode under standard conditions
Use of salt bridge
maintain charge balance
complete the circuit
Criteria for a salt bridge
does not react with either solutions in the half-cells
no precipitate will form with any ions in contact with it
(KNO3 is usually used as a salt bridge)
standard hydrogen electrode (SHE) and features
reference half-cell used for measuring standard electrode potential.
Consists of :
H2 gas at 1atm and 298K
platinum electrode
1moldm-3 H+ (aq) ion
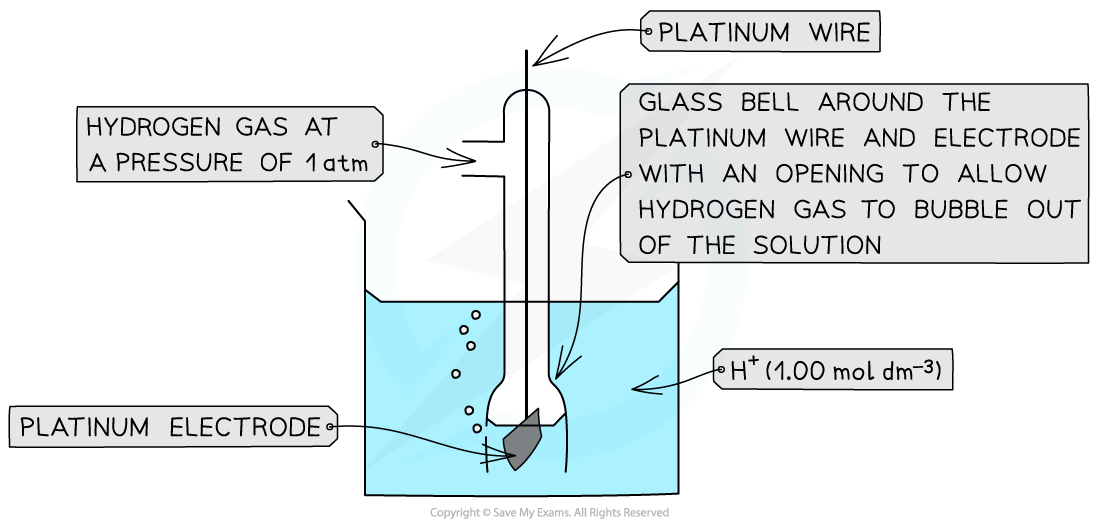
why is finely divided platinum electrode used in SHE
hydrogen does not conduct electricity so platinum is used to conduct and is inert so that it doesn’t react with H+ ions
finely divided to increase surface area for a faster reaction
A more positive E0 value implies that the ions are..
a greater oxidising agent
Standard cell potential (Ecell)
potential difference of a cell in a circuit between two half-cells under standard conditions
electron flow goes from…
more negative E0 to more positive E0 value
A reaction with a positive Ecell value is said to be..
feasible
why does the prediction of feasibility sometimes fail on positive Ecell value
Reaction has high activation energy
condition of the reaction are not standard
If a change causes the position of equilibrium to be shifted to the right, the value of E0 will…
increase
Nernst equation
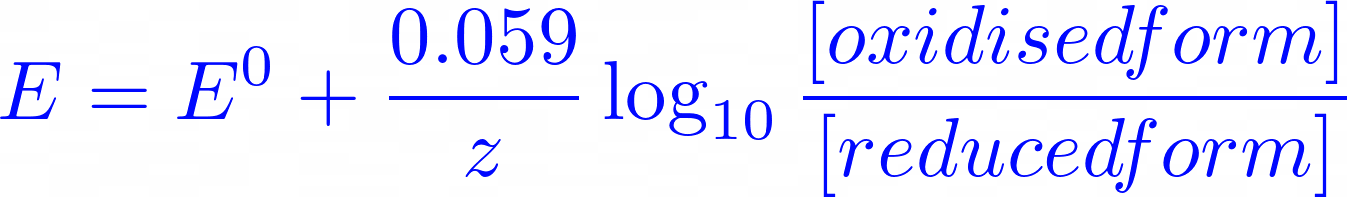
electrolysis
breakdown of an ionic compound by passing electric current
selective discharge of an ion is affected by :-
position of ion in electrochemical series
concentration of ion
Cations are selectively discharged when..
a more positive E0 (favours reduction more)
higher concentrtion (increasing E0 value)
why does an ionic compound have to molten during electrolysis
so that ions are free to move
why is graphite a suitable material for an electrode (two reasons)
conducts electricity
inert
hydrogen will be selectively discharged at the cathode unless…
the ions are gold,silver or copper
O2 will be selectively discharged at the anode unless…
the ions are halogens (it will discharge chlorine at a higher concentration)
formula of charge (Q)
Q=IT
1 Faraday
is the charge carried by one mole of electrons (96500C)
calculating 1 Faraday using avogadro constant
F=Le
L=avogadro constant
e = charge of one electron
Bronsted acid
proton donor
Bronsted base
proton acceptor
strong acid/base
one that completely dissociates/ionizes in water
weak acid/base
one that partially dissociates/ionizes in water
strong acid has a … conjugate base
weak
conjugate acid-base pairs
pair of reactants and products that are linked to each other by the transfer of a proton
Ka (acid dissociation constant) equation for
HA ⇌ H++A-
Ka= [H+][A-] / [HA]
the greater the Ka
the stronger the acid
pKa
pKa = -log10(Ka)
the lower the pKa
the stronger the acid
pH
pH = -log10[H+]
[H+]=10-pH
Kw value at 25oC and equation
Kw=[H+][OH-]
Kw = 1.0×10-14mol2dm-6 at 250C
explain why pH of a neutral solution is lower at higher temperature
H2O⇌H++ OH-
forward reaction is endothermic
so when temperature increases, equilibrium shifts to the right increasing concentrations of both H+ and OH- , since [H+] increases, the pH will decrease
but that does not mean that it is more acidic at higher temperatures since concentration of H+ and OH- are the same
strong acid [H+] formula
[H+] = [HA(aq)] (HA = strong acid)
(since the acid gets fully dissociated - it is assumed that the [H+] formed due to water is negligible and so is not included)
strong base [H+] formula
from Kw :
[H+] = Kw / [OH-]
(strong alkali have small amounts of H+ which are formed due to ionisation of water — we are dealing with aqueous solutions)
*remember to multiply concentration by x if molar ratio is 1:x
assumption made when deriving for pH of weak acids
1) ignore concentration of hydrogen ions produced by the ionisation of water
2) assuming the dissociation of weak acids is negligible (c-x approx = c)
calculation of [H+] for weak acids
[H+]=√(KaC)
C=concentration of acid
buffer solution
a solution which resists change in pH when small amounts of acid or alkali are added
Acid buffer
made up of a weak acid and one of its salts
Alkaline buffer
made up of a weak base and one of its salts
what happens when an acid is added to the buffer
(Assume dissociation is HA⇌H+ + A-)
[H+] added means equilibrium will shift to the left
a large supply of A- ensures that its concentration does not change rapidly (that supply comes from the salt — assuming its dissociation is NaA⇌Na++ A- )
a large supply of HA ensures its concentration does not change rapidly
so pH does not change significantly
pH of buffer solution
[H+] = Ka * [Acid] / [Salt]
pH = pKa + log( [Salt] / [Acid])
solubility
number of grams/moles of compound needed to saturate unit mass of water at a given temperature
Ksp
product of concentration of ions on a saturated solution of a sparingly soluble salt at 298K, raised to the power of their relative concentrations
common ion effect
reduction in the solubility of a dissolved salt achieved by adding a solution of a compound which has an ion common with the dissolved salt. which often results in precipitation
how to predict precipitation
if the product of the concentration is :
less than Ksp — no precipitate will be formed
more than Ksp — precipitate is formed
equial to Ksp — precipitate is not formed yet (limiting equilibrium ts)
* it causes equilibrium to shift to the left to the side of the solid so more solid is precipitated
Kpc
ratio of concentration of solute in two immiscible solvents at equilibrium at a particular temperature.
factors affecting Kpc
solubilities of the solute in two solvents
strength of imf between solute and solvents
polarity of the solute and solvents molecules
rate of reaction
rate of change in the concentration of a reactant or product per unit time
unit of rate (or rate of reaction)
moldm-3s-1
order of reaction (with respect to a reactant)
the power to which the concentration of that reactant is raised to in the rate equation
Zero order
rate = k[A]0
this means that the rate of reaction is independent on the concentration of A
Zero order :
rate-time graph
concentration of reactant-time graph
concentration of product-time graph


concentration of product just increases with +ve gradient passing through origin
First order
rate=k[A]
rate of reaction is proportional to the concentration of A
First order :
rate-time graph
concentration of reactant-time graph
concentration of product-time graph


*product concentration against time is just an increasing graph with decreasing gradient
half life
time taken for the initial concentration of reactant to decrease to half its original value
half life of a first order reaction is…
constant
relationship between half-life and rate constant is given by
K = ln(2) / t1/2
*only for first order