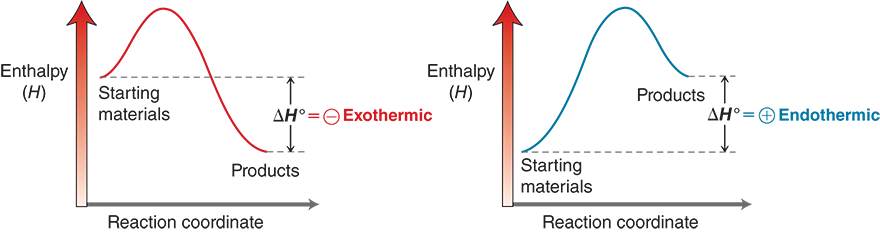
6.1 Enthalpy
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
How do electrons achieve a lower energy state?
Electrons achieve a lower energy state when they occupy a bonding molecular orbital rather than when they are isolated or in nonbonding/anti-bonding orbitals
Bond formation is favorable in lower energy states
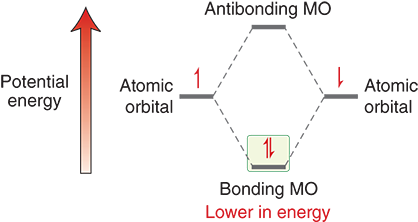
What happens to energy during bond formation?
energy is released
What happens to energy when bonds are broken?
energy is required
How is a bond broken?
To break a bond, electrons in the bonding molecular orbital must absorb energy from nearby molecules. These surrounding molecules transfer kinetic energy to the bond, giving the electrons enough energy to separate the atoms.
What is enthalpy?
A measure of the exchange of energy between the system and the surrounding
Δ𝐻=𝑞 (at constant pressure)
Δ𝐻= the change in enthalpy (the exchange of kinetic energy)
q=heat
What determines the ΔH of a bond-breaking reaction?
It is primarily determined by the amount of energy required to break the bond homolytically (each atom takes one electron).
More energy needed = larger ΔH (endothermic)
The ΔH of a bond-breaking reaction depends on the sum of all bonds broken and formed.
What is homolytic bond cleavage?
breaks a bond evenly, with each atom receiving a single electron and generates radicals
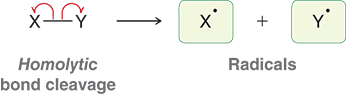
What are radicals?
A chemical entity with an unpaired electron
Ex:(hydroxyl radical OH and methyl radical CH3)
What is a single-headed arrow (fish hook arrow) used for?
A single headed curved arrow used in reaction mechanisms to show the movement of one electron
Used in radical reactions
Differs from regular (double-headed) arrows which show two electrons mpoving
Indicates single electron transfer or bond breaking\forming in radical mechanisms
What is a reaction mechanism?
A detailed, step-by-step explanation of how a chemical reaction occurs.
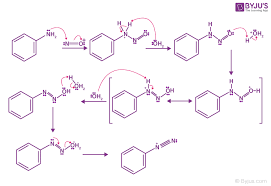
What is a heterolytic bond cleavage
breaks a bond unevenly, with both electrons going to one of the atoms
it is illustrated with a traditional, two headed curved arrow, generating ions
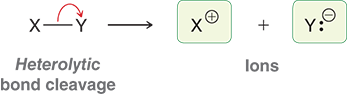
What are ions?
charged species
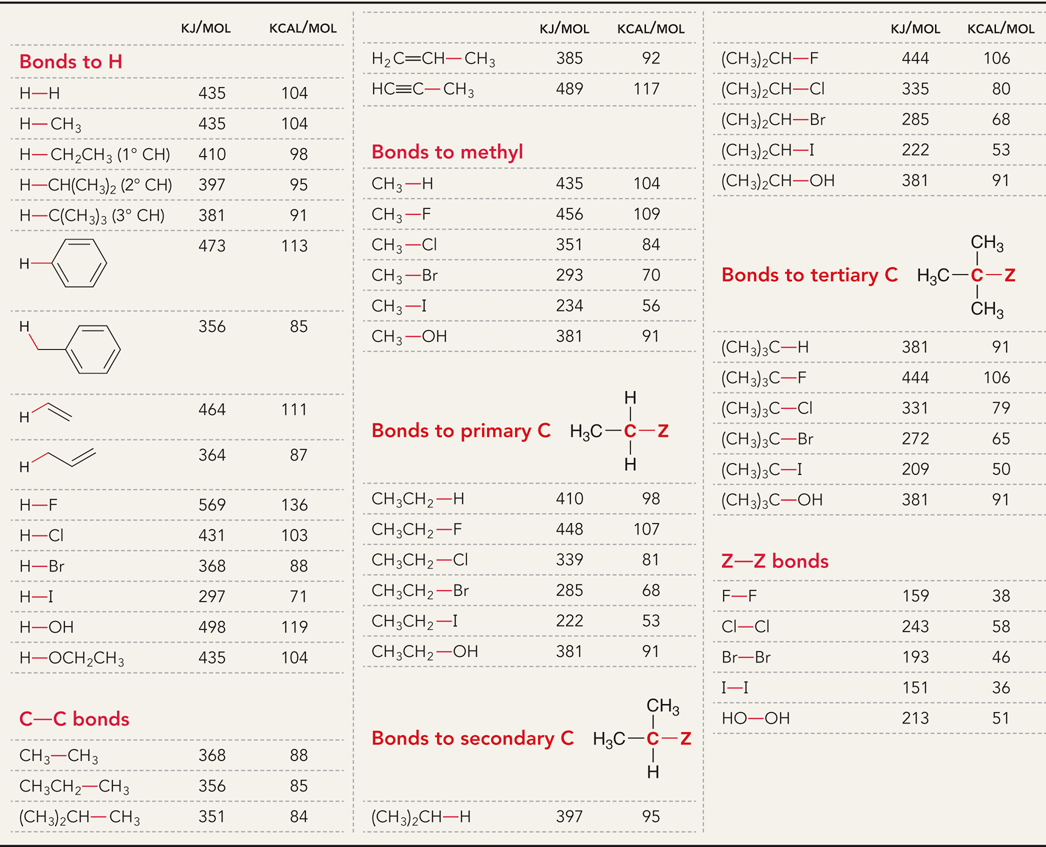
What is bond dissociation energy?
The energy required to break a covalent bond via homolytic bond cleavage
Δ𝐻° = refers to the bond dissociation energy when measured under standard conditions
Homolytically” just means each atom takes one electron when the bond breaks —
and bond dissociation energy measures the energy needed for that specific type of break.
Δ𝐻°: Table values
What is Primary (1°), Secondary (2°), and Tertiary (3°):
Refer to the number of alkyl groups attached directly to a given carbon atom
What is the heat of reaction
The heat given off during a reaction
Most reactions involve breaking and forming several bonds
The sign of ΔH° indicates whether energy is absorbed (+) or released (–) by the system.
What does it mean when something is positive H°
indicates that the system increased in energy
it received energy from the surroundings
What does it mean when something is negative H°
indicates the system decreased in energy
it gave energy to the surroundings
What does it mean when something is exothermic?
system gives energy to the surroundings'
ΔH° is negative
forming bonds
What does it mean when something is endothermic?
the system recieves energy from the surroundings
ΔH° is positive
breaking bonds
Two graphical representations plot enthalpy (H) on the vertical axis against reaction coordinate on the horizontal axis. In the first graph, a curve begins near three-fourth height of the vertical axis and before one-fourth of the length of the horizontal axis. This region is labeled, starting material. The curve increases in a concave upward manner, and reaches a peak at the top of the vertical axis and toward half the length of the horizontal axis. Thereafter, it gradually drops down and ends slightly above the one-fourth the height of the vertical axis and three-fourth the length of the horizontal axis, and this region is labeled, products. The enthalpy change, delta H superscript 0, equals negative exothermic. In the second graph, a curve begins near one-fourth height of the vertical axis and slightly away from the origin of the horizontal axis. This region is labeled, starting materials. The curve increases in a concave upward manner, and reaches a peak at the top of the vertical axis and half the length of the horizontal axis. Thereafter, it gradually drops down and ends at three-fourth the height of the vertical axis and three-fourth the length of the horizontal axis, and this region is labeled, products. The enthalpy change, delta H superscript 0, equals positive endothermic.