Standardise a solution of HCL using a standard solution of Na2CO3
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
Draw a diagram of the experiment
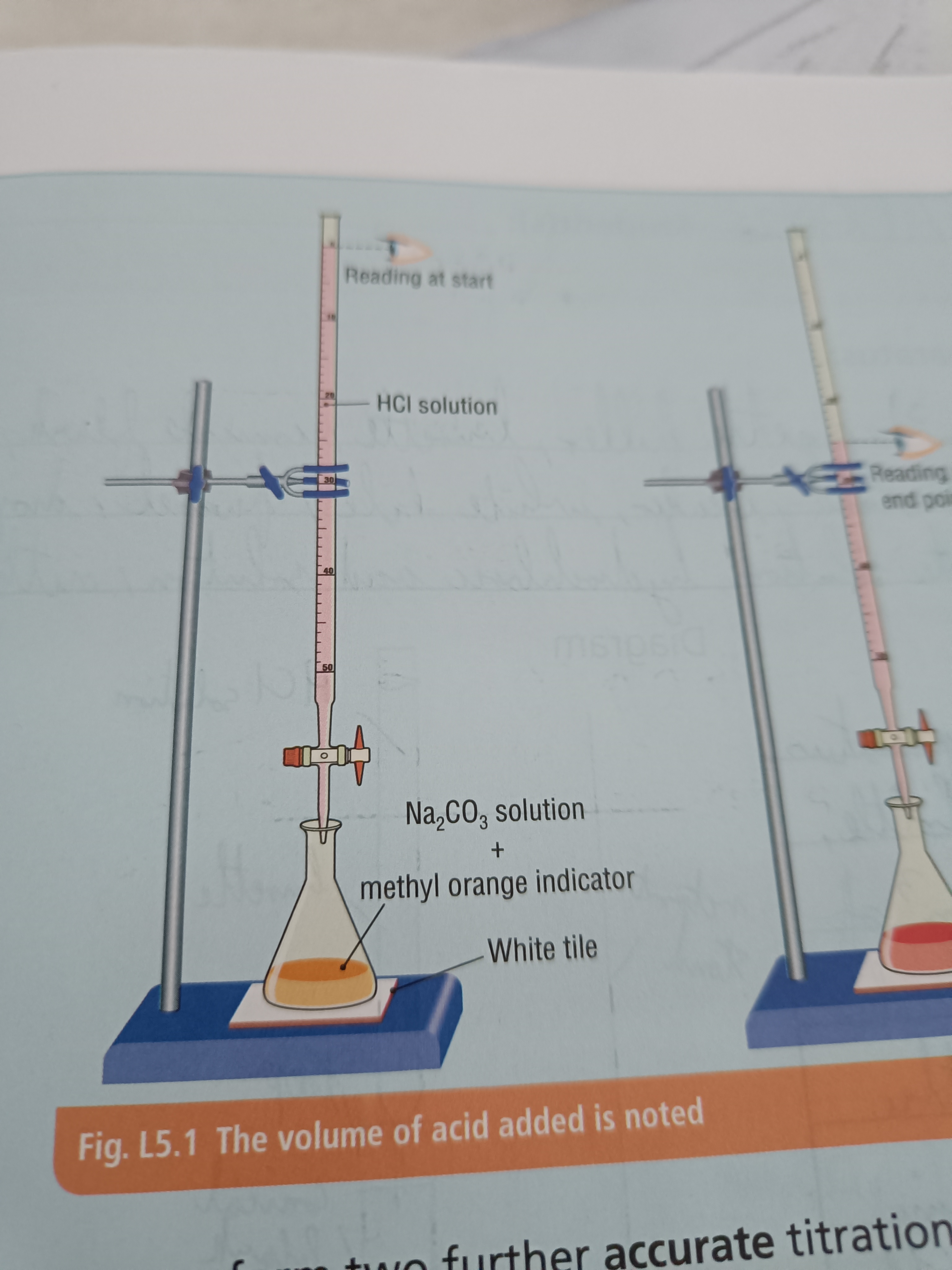
Step 1
Wash out the apparatus with a washbottle
Step 2
Pour acid into a beaker and repeat for the base
Step 3
Rinse out pipette and pipette Na2CO3 into a conical flask
Step 4
Add drops of methyl orange to conical flask
Step 5
Clamp burette with a retort stand and fill it with HCl using a funnel
Step 6
Open tap of burette into a spare beaker until HCl reaches the bottom of the meniscus
Step 7
Open tap again and swirl meniscus until solution turns from orange to pink
Step 8
Note volume of acid required and repeat 2 more times
Step 9
Calculate average from last two readings
Why is the first result of a titration ignored?
High chance it's inaccurate since it gives you the approximate end point
What gas is produced during this titration?
Carbon dioxide
Why does HCl have to be standardised?
HCl is not a primary standard
Why should you not add too much indicator?
They are weak acids/bases and too much could affect the results
Why shouldn't you pipette directly from the volumetric flask?
Impurities from pipette would contaminate entire solution in the volumetric flask