Biological molecules
1/115
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
116 Terms
What does δ+ mean What does δ- mean
Slight positive charge Slight negative charge
What is the meaning of a polar molecule
it has an uneven distribution of charge that gives it both positively charged and negatively charged areas.
What is hydrophobic?
Non-polar and uncharged molecules (or parts of molecules) do not bind to water molecules and are called hydrophobic.
What is hydrophilic
Hydrophilic is a term used to describe molecules (or parts of molecules) that readily bind to water and so are attracted to water molecules. Being polar or charged makes a molecule (or ion) hydrophilic.
What is a monomer
A molecule that is a basic single unit of a large chain-like molecule.
What is a polymer
A large molecule that is composed of many single units (that are identical or very similar) joined together.
What is a Dimer
A molecule that is composed of two single units (that are identical or very similar) joined together.
What is an oligomer
A molecule that is composed of several single units (that are identical or very similar) joined together.
What is a condensation reaction
Reactions that join molecules together with the release of water molecules.
What is a hydrolysis reaction
Reactions that break larger molecules into smaller molecules using water molecules.
What is metabolism?
All the chemical reactions that go on in an organism.
Carbohydrate is a category of biomolecule •what elements does it contain? •what are its building blocks or monomers •Name is dimer? •Name it's macromolecule? •Is it a polymer? •Name it's bond •Name the joining reaction? •Name the splitting reaction? •Functions?
Elements= carbon, oxygen and hydrogen
•Building blocks or monomers = monosaccharides eg: glucose
•Dimer = Disaccharide eg: sucrose
•Macromolecule = polysaccharide
• It's a polymer
•Bond = glycosidic
Joining reaction= condensation
Splitting reaction = Hydrolysis
Functions =Energy storage and supply, structure in some organisms
Protein is a category of biomolecule •what elements does it contain? •what are its building blocks or monomers •Name is dimer? •Name it's macromolecule? •Is it a polymer? •Name it's bond •Name the joining reaction? •Name the splitting reaction? •Functions?
Elements = carbon, hydrogen, oxygen, nitrogen, sulphur
•Building blocks = Amino acids
•Dimer = Dipeptide
•Macromolecule = polypeptide/protein
•Its a polymer
• bond = peptide
•Joining reaction = condensation
•splitting reaction = hydrolysis
•Functions = Structure, transport, enzymes, antibodies
Lipid (only focusing on fats and oils) is a category of biomolecule) what elements does it contain? •what are its building blocks or monomers •Name is dimer? •Name it's macromolecule? •Is it a polymer? •Name it's bond •Name the joining reaction? •Name the splitting reaction? •Functions?
Elements = carbon, hydrogen, oxygen
•building blocks/ monomers = fatty acids and glycerol
•Dimer = none
•macromolecule = triglyceride
• no it's not a polymer
• bond = ester
•joining reaction = condensation
•splitting reaction = hydrolysis
• Functions = membrane energy supply, thermal insulation
Nucleic acid is a property of biomolecule what elements does it contain? •what are its building blocks or monomers •Name is dimer? •Name it's macromolecule? •Is it a polymer? •Name it's bond •Name the joining reaction? •Name the splitting reaction? •Functions?
Elements = carbon, oxygen, nitrogen, hydrogen, phosphorus
•building blocks = nucleotides
•Dimer =Dinucleotide
• macromolecule = nucleic acid eg: DNA/ polynucleotide
• Yes it is a polymer
•Bond=phosphodiester
•Functions = storage, processing on genetic information, protein building
What is an oligosaccharide? How many monomers does it have
Medium length carbohydrates 2-20 monomers
Properties of monosaccharides and disaccharides
Water soluble (because has a large number of OH groups or hydroxyl groups which can form hydrogen bonds with water molecules) •will have osmotic effect •Diificult to store water if present •can be transported through fluid phloem
Sweet •Used as an attractant to animals eg: fruit
Form crystals •can be stored in the absence of water eg: sugar cane or beets
Give types of monosaccharides and their formula
Triose= C3H6O3 Tetrose C4H8O4 Pentose = C5H10O5 Hexose = C6H12O6
Examples of pentose sugar Give uses of pentose sugar
Ribose
Examples of hexose sugar? Give uses of hexose sugar?
Glucose
What is a structural isomer
have the same chemical formula
How are carbon molecules arranged in glucose molecule
Clockwise
What is the difference between an alpha glucose and beta glucose
The OH group on carbon 1 of a-glucose is below the ring
Why is glucose an important molecule
Glucose is polar
State and explain how a glucose molecule is well suited to its function in living organisms
Soluble so it's easily transported around organism Small molecule so can be transported across cell membrane Easily broken down to release energy Molecules can join to produce disaccharides or polysaccharides
How do monosaccharides go to disaccharides
Condensation reaction
What is the bond between monosaccharides
glycosidic bonds
What is a glycosidic bond?
a covalent bond formed between 2 monosaccharides by a condensation reaction
What do 2 alpha glucose molecules combine to make and what is its bond
Maltose Alpha 1
What does an alpha glucose molecule and a fructose molecule combine to make and what is its bond
Sucrose Alpha 1
What does a beta glucose molecule and a galactose molecule combine to make and what are its bonds
Lactose Beta 1
Types of disaccharide and uses
Sucrose: storage = sugar cane or beet
Similarities between lactose and maltose
Both hexose sugars Both contain 1-4 glycosidc bonds
Differences between lactose and maltose
Lactose = has one glucose monomer and one galactose monomer
How are condensation and hydrolysis reactions important
Condensation reactions are important for building up large molecules for storage eg: glucose to glycogen
Hydrolysis reactions are important to breakdown large insoluble molecules into smaller soluble molecules
What two polysaccharides make up starch
amylose and amylopectin
Amylose
Where is it found
What monomer is it made of
Does it have branches
Does it have 1,4 glycosidic bonds
Does it have 1,6 glycosidic bonds
Is it soluble
Structure / function
Plant
Alpha glucose
No
It has 1,4 glycosidic bonds
No
It is soluble
Function / structure =too large to diffuse through cell membrane and pass out of cell , amylose coils to form a tight helix and this makes starch compact. Starch can store a large amount of glucose molecules for its size. (Stores a lot of energy)
Amylose only has 2 accessible ends what does this mean
It is broken down slowly by enzymes so there is a slow release of alpha glucose
What happens to amylose complex of heated
Hydrogen bonds break Causing helix to unravel
Amylopectin
Where is it found?
Made from what monomer
Branches
1,4 glycosidic bonds
1,6 glycosidic bonds
Soluble
Structure / function
Plant
Alpha glucose
Yes per 20 subunits
Yes
Yes
It is insoluble
Structure and function= Chains form in to helix, Large number of branches mensing it has ends so the enzymes break down starch rapidly, there energy is released quick. Too large to diffuse through cell membrane and pass out of cell
How is amylopectin different to amylose
Amylopectin is a heavily branched molecule
As amylopectin has 1-6 glycosidic bonds it enables it to have more side branches and accessible ends what does this mean
Quicker release of alpha glucose so it is more easily broken down
Glycogen
Where is it found
What monomer is it made of
Does it have branches
Does it have 1,4 glycosidic bonds
Does it have 1,6 glycosidic bonds
Is it soluble
Structure / function
Animals (liver and muscle cells)
Alpha glucose
Yes per 10 subunits (heavily branched)
Yes
Yes
Insoluble ( doesn't draw water into cells by osmosis )
Function /structure = chains to form into a helix similar structure to amylopectin, so it's compact has lots of branches so has free ends so enzymes can convert glycogen back to glucose very rapidly. As it can convert it to glucose rapidly it can be used in respiration. Eg: as animals have a high metabolic rate it gives them animals energy to escape predators
Why does glycogen make a good storage molecule
Insoluble Doesn't affect water potential Lots of branches for enzymes to attach Compact so can store a high amount of energy Can be broken down and built up quickly
Cellulose
Where is it found
What monomer is it made of
Does it have branches
Does it have 1,4 glycosidic bonds
Does it have 1,6 glycosidic bonds
Is it soluble
Functions/ structure
Plant
Beta glucose
No
Yes
No
Insoluble
Structure/function = straight chains that are close to each other, Has
microfibrils which are strong threads made of long cellulose chains and they provide structural support, has many hydrogen bonds making it strong
Microfibrils can also bundle together creating macrofibrils which provide extra strength
Important in human diet it provides a fibre to keep digestive system healthy + herbivores eat a lot of plants so it can be diverse and converted into energy quickly.
Properties of cellulose that make it suitable for forming cell walls
Insoluble High tensile strength Has microfibrils or macrofibrils
How are beta glycosidic bonds formed in cellulose
Every other beta glucose molecule must be flipped 180° (upside down)
Differences structurally between amylose and cellulose
Amylose = coiled
What things can be reducing sugars
All monosaccharides and the disaccharides maltose and lactose
Name something that is a non reducing sugar
Sucrose
How to test for starch
•Place sample in clean test tube •Add iodine solution and gently shake •If starch is present the solution changes from a yellow- brown to a blue-black
what are the groups of a lipid molecule in displayed formula
1 molecule of glycerol 3 molecules of fatty acids
How do you test for lipids
Add sample to clean test tube and add ethanol/alcohol If a white cloudy emulsion forms lipids are present
What type of molecule is a lipid
macromolecule - not a polymer
Describe the structure of triglycerides
~ Have one molecule of glycerol with 3 fatty acids attached ~ ester bond formed between glycerol and fatty acid in condensation reaction Has 3 hydroxyl groups Has carboxyl (COOH) group In the hydrocarbon of fatty acids we can replace the long chain with R
What is an ester bond
A covalent bond formed by condensation reaction between OH group of a carboxylic acid and OH group of alcohol
Are lipids polar or non-polar?
non-polar - not dissolve in water
Fats are what at room temperature Oils are water at room temperature
Solids Liquid
Fatty acid molecules with hydrocarbon chains with no double covalent bonds between carbon atoms are what?
Saturated
Fatty acid molecules with hydrocarbon chains that do have double covalent bonds between carbon atoms are called what
Unsaturated 1 = monounsaturated More than 1 = polyunsaturated
How is a phospholipid molecule different to a triglyceride
One of the fatty acid molecules is replaced with a phosphate molecule on the other side
The phosphate and glycerol molecule on a phospholipid is called what
The head
The fatty acids on a phospholipid molecule is called what?
Tails
The negative charge means the phospholipid head is what
Hydrophilic - attracted to water due to having a charge
If the phospholipid head is hydrophilic what are the tails
Hydrophobic - they are non polar / not charged it is repelled by water
Phospholipids form a layer on water with its head towards water and tail away from water true or false
True
Phospholipids form a mono layer of phospholipids called what
Micelles - water is surrounding it so hydrophilic head is faced towards water but hydrophobic tail is in core of Micelle where there is no water
How do phospholipids form a bilayer
2 layers
In cells where are phospholipid bilayers involved in
Internal membranes eg: Golgi apparatus + lysosomes Cell surface membrane
How do phospholipids keep membrane fluid
Can move past each other
How does the fluidity of phospholipids change
In unsaturated molecules the tails gave more fluid In saturated molecules the tails have less fluid
Describe structure of cholesterol
Four carbon rings joined a type of steroid Synthesised in liver and transported via blood
Needed for cell membranes brain and nerve tissue and vitamin D
High levels of it can clog arteries by forming plaques and may contribute to cardiovascular disease
Cholesterol has hydrophilic and hydrophobic regions true or false
True - exits as a bilayer as well
What is cholesterol used in
Molecules of cholesterol are used to produce steroid-based hormones such as oestrogen
what are the groups on a protein in displayed formula
Amine group NH2 carboxyl group COOH Hydrogen group H R group
Functions of proteins
Structure = main component of body tissues
Catalytic = all enzymes are proteins
Signalling = many hormones and receptors are proteins
Transport = proteins are a major element in transport and storage
Defence = all antibodies are proteins
How is a peptide bond formed between amino acids
The OH of a carboxylic acid and the H of an amine group on the next amino acid
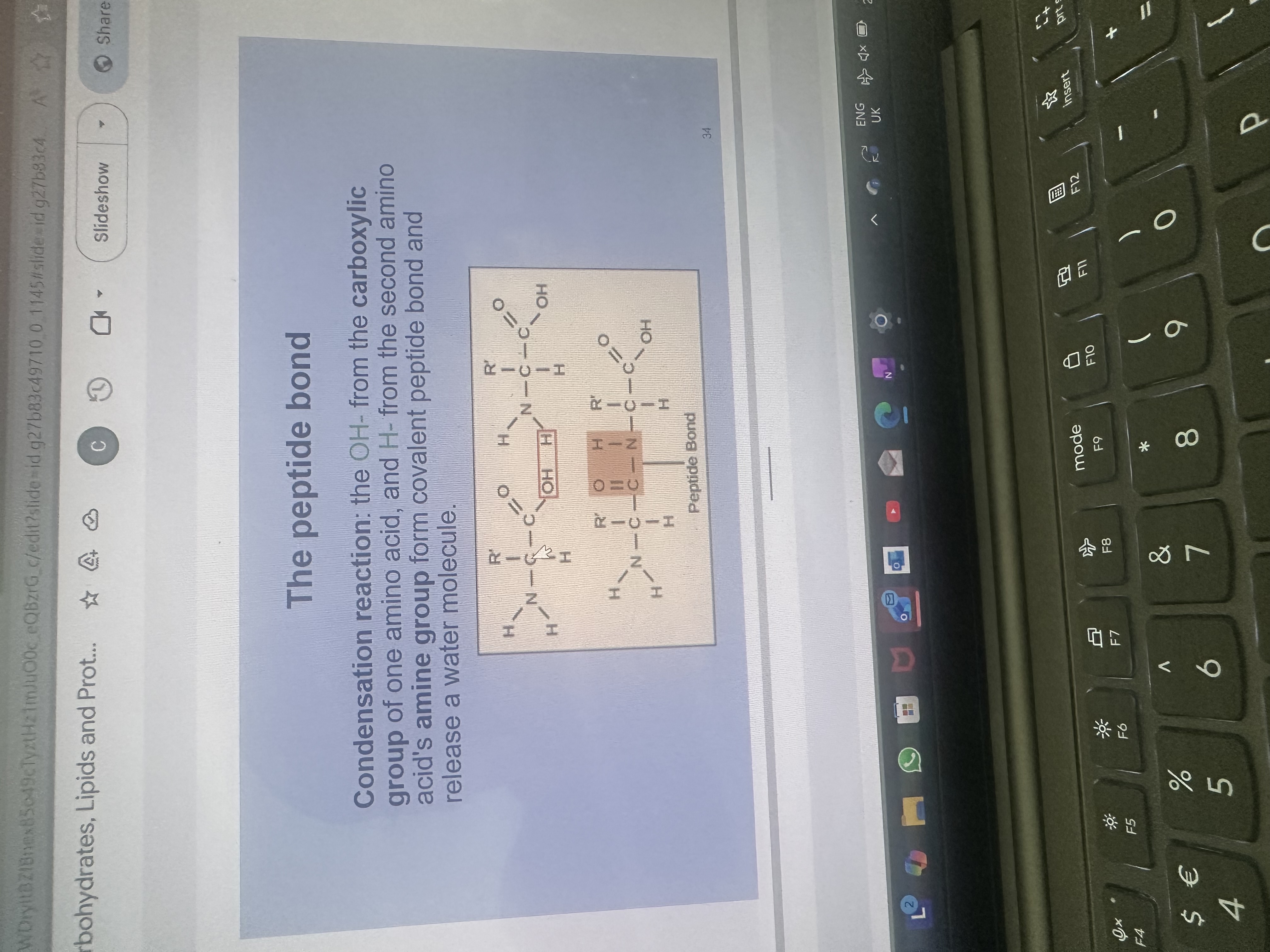
Are amino acids monomers or polymers
monomers
Are proteins monomers or polymers?
polymers
How many naturally occurring amino acids have different R groups
20
Every protein has its own unique shape true or false
True
What is the primary structure of a protein?
sequence of amino acids
Difference between a polypeptide and protein
Polypeptide is joining of amino acids that can curl into 2 different shapes A protein is the joining of amino acids
2 shapes polypeptides form
Alpha helix
Beta pleated sheet
What is secondary structure
Curling or folding of polypeptide chain into alpha helix and beta pleated sheets due to hydrogen bonds
What is a alpha helix
Polypeptide chains coil with hydrogen bonds keeping coil stable Hydrogen bonds occur between C and O and H and N
What are beta pleated sheets
The chains form zig zags and fold over themselves in rows parallel to each other and hydrogen bonds form
Hydrogen bonds keep secondary structure stable true or false
True
What is a Tietiary structure
Overall specific 3d shape of a protein. This is determined by interactions between R groups and properties of R groups
What are tiertiary structures composed of
•Alpha helices and beta pleated sheets integrate to form 3d structure •There are hydrogen bonds between R groups ( as long as R groups are polar ) •Ionic bonds are formed between positively and negatively R groups •Disulphide bonds between R groups and sulfur
Tiertiary 3d structure can also be determined by what
Hydrophilic and hydrophobic R group
What other structure determines the tiertiary structure
Primary structure
What is the quanternary structure
Specific 3d shape of a protein that is determined by multiple polypeptide chains and prosthetic groups bonded together.
What is a quaternary structure composed of
•Made up of multiple polypeptide chains •held by hydrogen
Factors affecting protein structure
PH
Salt concentration
Temperature
Other environmental factors
3d structure determines what
If a protein is globular or fibrous
What is a globular protein
A protein with a spherical shape that is soluble in water and typically has metabolic roles
What is Haemaglobin
A globular transport protein found in red blood cells
Role of haemoglobin
To transport oxygen from lungs to body tissues
Apart from it being a globular protein what type of protein is haemoglobin
Conjugated protein