EVSC Module 10
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
Ocean Acidification
Climate change isn’t the only consequence of carbon pollution.
With increasing carbon dioxide in the atmosphere, oceans absorb more and more of it, becoming increasingly acidic.
This is happening at an unprecedented rate and will continue unabated if we don’t stop burning fossil fuels
Ocean (Marine) Ecosystems
Ocean ecosystems cover 70% of Earth’s surface, and house a greater variety of flora and fauna (plants and animals) than all land masses combined
Coral reefs
Coral reefs are large, underwater structures formed by colonies of small invertebrates (corals), that produce calcium carbonate exoskeletons which accumulate over time
Approximately 25% of all ocean species spend some portion of their life in a coral reef. This important role in marine ecology makes monitoring coral reefs critical to understanding how ocean ecosystems function
Corals
Considered the ultimate keystone species because they provide the underlying structure for reef communities.
Ocean Absorption of CO2
solubility cycle
biological cycle:
photosynthetic organisms convert co2 into organic matter via photosynthesis
solubility cycle
Co2 dissolved into water, increasing Pco2.
dissolve co2 then reacts with the water to form carbonic acid.
carbonic acid further disassociates to form bicarbonate Hco3, and free hydrogen ions
the hydrogen ions are what the ocean more acidic.
Calcium Carbonate comes from shells of organisms
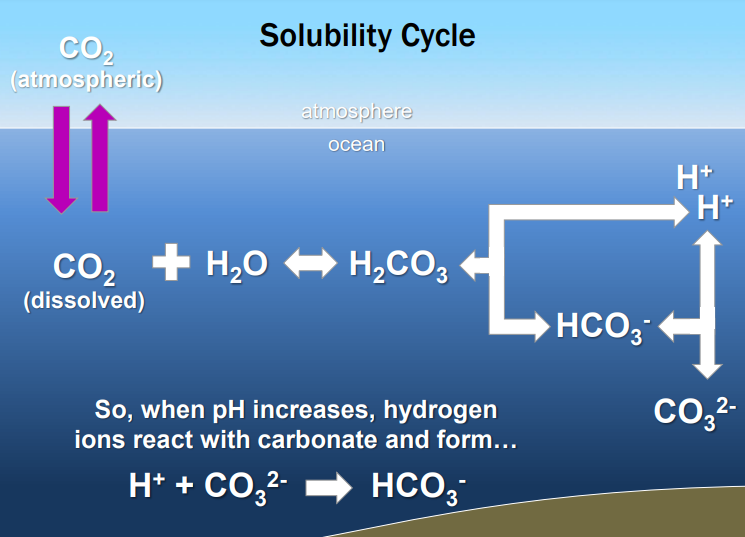
Since the ocean carbon cycle can buffer against changes in pH, we should not see any change in seawater pH as a result of anthropogenic input of CO2.
False
The solubility cycle can’t buffer all of it, which is why we are seeing global decreases in ocean pH
Since we are creating two hydrogens for every co2 were putting in
Ocean Acidification in Numbers

Planetary Boundary
less than or equal to 80%
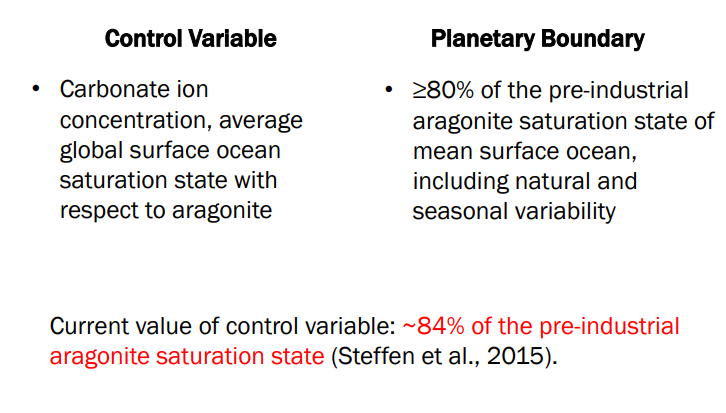
What is “Saturation State with respect to aragonite”?
