MET n (MET 03) Briefing, debriefing & pilot studies
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
Explain the purpose of using a briefing in research (2)
* To obtain informed consent from pps
* To inform them of anything that might affect their willingness to participate
Explain the purpose of using a debriefing in research (3)
* To complete the pps understanding of the study
* To obtain permission (retrospective consent) to use their data
* To identify any potential negative effects on pps and offer to support hem if necessary
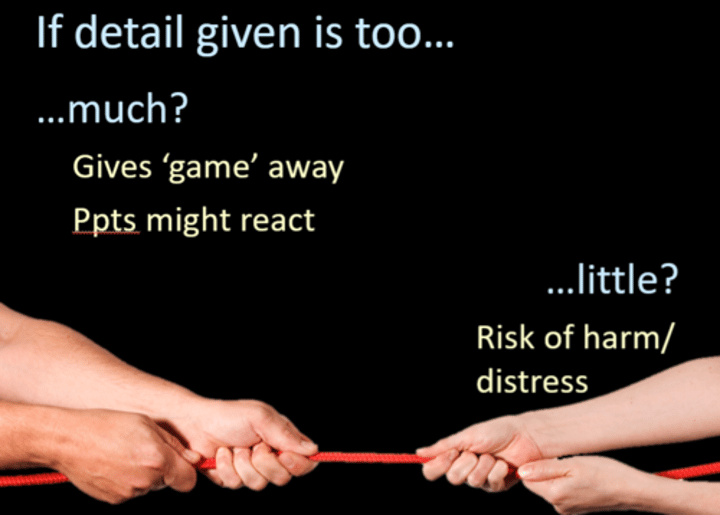
Explain the importance of balancing consent and validity in a briefing (2)
* Must obtain informed consent for ethical reasons...
* ...but too much information could increase demand characteristics and reduce validity
Explain what a pilot study is and what it involves doing (5)
• Small scale version of investigation
• Occurs before the real investigation to pre-empt any problems
* Ensure the study is valid, practical and ethical
• Check that the procedure, materials, measuring scales /equipment work well
• Therefore, researcher can identify potential issues and fix them, saving time and money
Explain some things that can be checked during pilot studies using experiments, self- reports and observations (3x2)
Experiments
* Check that experimental tasks are clear...
* ...and work properly, timings are right
Self-report methods
* Help re-word ambiguous questions if misleading
* Train researchers, esp interviewers
Observations
* Check coding systems
* Train observers