Microbio Chapter 11: Catabolism
1/68
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
69 Terms
What do phototrophs use
light
What do chemotrophs use
oxidation of chemical compounds
What do organotrophs use
organic compounds
What do lithotrophs use
reduced inorganic substances
What do heterotrophs use
organic molecules
can also be used for energy source
What do autotrophs use
a single carbon molecule
usually carbon dioxide
What are the major classes microorganisms
photolithoautotrophs / photoautotrophs
primary producer
chemolithoautotrophs
primary producer
chemoorganoheterotrophs / chemoheterotrophs / chemoorganotrophs
same organic nutrient can satisfy all three requirements
majority of pathogens
What are primary producers
organisms that produce things that other organisms are going to use
What are the basic needs that all organisms have
ATP as an energy currency
reducing power to supply electrons
NADH and FADH2
adds H atoms
precursor metabolites to provide carbon skeletons
converted into monomers which are polymerized into macromolecules
What can chemoorganotrophs do
fermentation
aerobic respiration
anaerobic respiration
What can chemolithotrophs do
aerobic respiration
anaerobic respiration
Where are electrons donated during respiration
ETC
Where are electrons donated during fermentation
an endogenous acceptor
What does respiration involve the use of
an ETC
Aerobic respiration
oxygen is the final electron acceptor
anaerobic respiration
exogenous acceptor is used
NO3-, SO42- , CO2, Fe3+, or SeO42-
What is generated during respiration
pmf
What does pmf fuel
oxidative phosphorylation to generate ATP
What does fermentation use
an endogenous electron acceptor
so it needs an organic compound
ex. pyruvate
What does fermentation not involve
the use of an ETC or the generation of a PMF
No OP
How is ATP synthesized
only by substrate-level phosphorylation (SLP)
ADP + P —> ATP
What are the important carbon skeletons from EMP
G6P
F6P
G3P
3PG
PEP
Pyruvate
What are the important carbon skeletons from PPP
Erythrose-4-phosphate (E4P)
Ribose-5-phosphate (R5P)
What are the important carbon skeletons from TCA
Acetyl CoA
Alpha-ketoglutarate
Succinyl CoA
Oxaloacetate
What is the overall goal of aerobic respiration
to completely catabolize an organic energy source to CO2
What are the 3 steps in aerobic respiration
Glycolysis: glucose —> pyruvate
EMP, ED, and PPP
TCA: pyruvate —> CO2
ETC: O2 is the final electron acceptor
generates pmf which fuels OP to produce ATP
Where are metabolic pathways located in prokaryotes
glycolysis: cytoplasm
TCA: cytoplasm
ETC: inner/plasma membrane
Where are metabolic pathways located in eukaryotes
glycolysis: cytoplasm
TCA: mitochondrial matrix
ETC: inner mitochondrial membrane
What do all the glycolytic pathways have in common
provide precursor metabolites to all other pathways
glucose → G3P
G3P → pyruvate
oxidized in the same way in all pathways
What is the most common pathway for glycolysis
Embden-Meyerhof Pathway (EMP)
Embden-Meyerhof Pathway (EMP)
functions in the presence or absence of O2
two phases
6 Carbon phase (uses 2 ATP)
forms F1,6BP
3 carbon phase (makes ATP)
F1,6BP → (2) G3P
Net ATP gain/glucose: 2 ATP
What is the net ATP gain per glucose molecule in the EMP
2 ATP
Entner-Doudoroff Pathway (ED)
used by Gram- soil bacteria
not by eukaryotes
many under aerobic conditions
E. coli and Enterococcus faecalis
replaces first phase of the EMP
2-keto-3-deoxy-6-phosphogluconate (KDPG) → pryuvate + G3P
Net yield / glucose: 1 ATP + 1 NADH + 1 NADPH
What is the net yield per glucose molecule in the ED pathway
1 ATP + 1 NADH + 1 NADPH
What is another name for the Pentose Phosphate Pathway (PPP)
hexose monophosphate pathway
What does the PPP do
oxidize G6P → → Ribulose-5-phosphate + CO2
The Pentose Phosphate Pathway (PPP)
not oxygen dependent
works simultaneously with ED or EMP
anaerobic or aerobically
not found in Archaea
found in both eukaryotes and bacteria
needed for biosynthesis and catabolism
major source of NADPH (anabolism)
Produces E4P and R5P
intermediates used to generate ATP
can be degraded into pyruvate by EMP
can regenerate G6P by gluconeogenesis
Amphibolic Pathways
functions in both anabolic and catabolic process
depends on levels of ATP, PEP, and F6P
Includes
EMP/gluconeogenesis
TCA cycle
PPP
What is another name for the TCA Cycle
Citric Acid Cycle or Krebs Cycle
How many times does glucose have to go through the TCA
twice
Where does the TCA cycle occur
in the cytoplasm
What is the source of carbon skeletons for biosynthesis
TCA
What does the TCA cycle produce
2 CO2, 3 NADH, 1 FADH2, and 1 ATP per acetyl CoA
Where is most of ATP made
the ETC
What is the DE’0 between NADH and O2
1.14 V
What is the ETC
a series of electron carriers
from more negative reduction potentials to more positive
What happens as electrons move through the mitochondrial ETC
coupling sites move H+ across the inner mitochondrial membrane → pmf
pmf powers OP to make ATP
What are the different complexes of the mitochondrial ETC
Complex I - NADH-ubiquinone oxidoreductase (NADH dehydrogenase)
coupling site
CoQ connects complex I to III
Complex II - succinate dehydrogenase
CoQ connects complex II to III
Complex III - ubiquinol-cytochrome c oxidoreductase
coupling site
Cyt c connects complex III to IV
Complex IV - cytochrome c oxidase
coupling site
transfers e- to O2
Prokaryotic vs Eukaryotic ETCs
Location
prokaryotes: inner/plasma membrane
eukaryotes: IMM
Different e- carriers
may be branched
may be shorter
lower P/O ratio
how much NADPH produces ATP from oxygen available
number of ATP synthesized per oxygen atom reduced
Escherichia coli ETC
facultative anaerobic bacterium
can do fermentation
branched pathway dependent on oxygen levels
bd branch - stationary phase and low aeration
higher affinity for oxygen
moves fewer protons
bo branch - log phase and high aeration
lower affinity for oxygen
H moves from cytoplasm to periplasmic space creating pmf
uses ubiquinone as e- carriers
Paracoccus denitrificans ETC
facultative anaerobic soil bacterium
non-fermenting
can use aerobic respiration similar to mitochondrial ETC
similar electron carriers
protons transported to periplasmic space
extremely versatile
both hetero and autotrophic
glucose: uses NADH to donate e- to NADH dehydrogenase
e- enter Complex I = pumps more protons out
1-carbon molecule (methanol): no NADH involved, e- donated to cyt c via methanol dehydrogenase (MD)
e- enter Complex IV = pumps less protons out
How do protons move in the mitochondrial ETC
from the matrix to the intermembrane space
Where is the F1F0 ATP synthase found
mitochondria, bacteria, and chloroplast
F1F0 ATP synthase
best studied ATP synthase
can also catalyze ATP hydrolysis
F0 is the proton conducting channel
goes through membranes
protons go across
rotates like a fan
F1 is a complex that catalyzes ATP synthesis/hydrolysis
What is the theoretical maximum yield of ATP during aerobic respiration
32 ATP
maximum in eukaryotes is 30 ATP
less in prokaryotes due to shorter ETC and lower P/O
How to calculate maximum ATP yield
using P/O ratios of NADH (2.5) and FADH2 (1.5)
Anaerobic Respiration
exogenous electron acceptor other than O2
yields less energy d/t lower reduction potential of acceptor
done by all 3 domains
most common electron aceptors: nitrate, sulfate, and carbon dioxide
Paracoccus denitrificans anaerobic respiration
anoxic conditions: dissimilatory nitrate reduction/denitrification
NO3- → NO2- → NO → N2O → N2
enzymes are inhibited by O2
because aerobic respiration yields more energy than anaerobic
nitrate as terminal electron acceptor → N is unavailable to cell for assimilation or uptake
causes loss of soil fertility
also done by Pseudomonas and Bacillus (facultative anaerobes)
Fermentation
energy source is only partially oxidized
less ATP per glucose
NADH produced by glycolysis → NAD+
pyruvate or derivative accepts electrons
oxygen is not needed
No ETC
OP does not occur
ATP is formed by SLP only
no pmf
Common Microbial Fermentation
Fermentation pathways are named after what’s produced
Mixed acid fermenters (E. coli)
Butanediol fermenters (Enterobacter)
Alcoholic acid fermenters
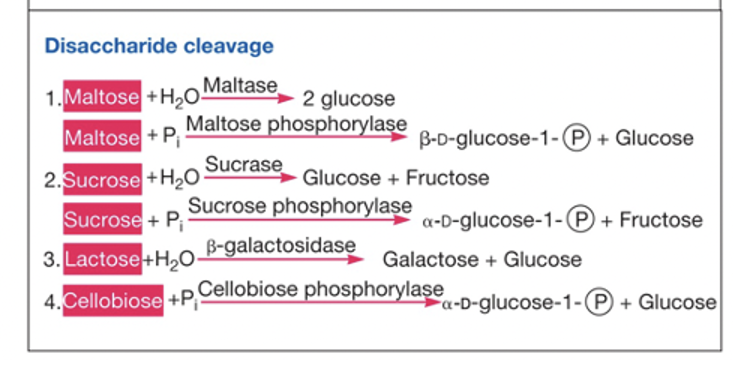
Catabolism of Carbohydrates
Carbohydrates can be supplied externally or internally
disaccharides and polysaccharides are cleaved into monosaccharides
hydrolases (outside the cell)
uses water
phosphorylases (inside the cell)
adds a phosphate to one of the products to use less ATP
yields G1P to enter glycolysis after conversion to G6P
Lipid Catabolism
used by chemoorganotrophs
hydrolyzed by lipases to
glycerol degraded via glycolytic pathway as dihydroxyacetone phosphate (DHAP) → G3P
fatty acids oxidized via B-oxidation
shortened by two carbon units that are released as acetyl-CoA
which is then fed into the TCA cycle or for biosynthesis
Protein and Amino Acid Catabolism
Proteases hydrolyzes protein to amino acids (proteolysis)
Deamination followed by transamination
resulting in organic acids converted to pyruvate, acetyl-CoA, or TCA cycle intermediate
Chemolithotrophy
e- released from inorganic molecule
common energy sources are: H2, reduced nitrogen, reduced sulfur, and Fe2+
directly donate electrons to ETC
Terminal electron acceptor
oxygen, sulfate, and nitrate
Must use CO2 as carbon source
CO2 fixation pathways
ETC is used, ATP is synthesized by OP
they do NOT use fermentation
Three major groups of chemolithotrophs
several bacteria and archaea oxidize hydrogen
reduces NAD+ or donate directly to the ETC
Nitrifying bacteria carry out nitrification
oxidation of ammonia (NH3) to nitrate (NO3-)
Step 1: ammonia → nitrite
Step 2: nitrite → nitrate
Sulfur-oxidizing microbes
oxidizes hydrogen sulfide (H2S), sulfur, and thiosulfate (S2O32-) to sulfuric acid (H2SO4)
Reverse Electron Flow
used by chemolithotrophs which many of them are autotrophs
needs NAD(P)H and ATP to reduce CO2
but they cannot donate electrons directly to NAD(P)+ so they use reverse electron flow
during reverse electron flow, electrons are moved up their ETCs to reduce NAD(P)+ to NAD(P)H
energy from H+ going from out to in (pmf) helps electrons move up the E0’ tower
Chemoorganotrophic fueling process
energy source is oxidized
releases electrons that are accepted by NADH/FADH2
releases energy (catabolism) and provides carbons and electrons needed for anabolism
Work without ETC
pmf is still needed for other functions
fermentation: end-products efflux
products go from high concentration to low concentration produced energy
facultative anaerobes: pmf redox-loop mechanism
strictly fermentative conditions: F1F0-ATP synthase operates reversibly
ATP → ADP
pumps protons out
Disaccharide cleavage