Lecture 1: Properties of Seawater
1/27
Earn XP
Description and Tags
Midterm 1
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
Properties of seawater:
High boiling and freezing points.
High heat capacity.
Large latent heat of vaporization.
Molecules are cohesive.
Universal solvent.
Liquid form denser than solid.
Density varies with temperature and salinity.
Low viscosity.
Sound travels far, but light doesn’t
Properties of seawater: Importance
Distribution of oxygen and carbon in the planet.
Relatively stable conditions for life on Earth.
Ocean circulation.
Climate
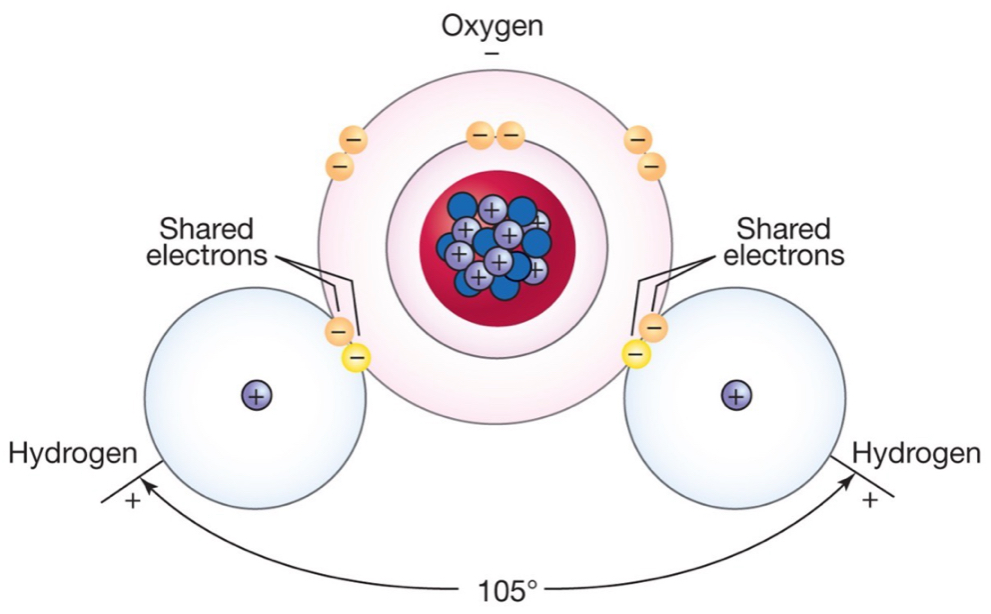
Water molecule structure:
Oxygen atom (8 electrons) about twice the size of hydrogen (1 electron).
Covalent bond between oxygen and hydrogen atoms.
Relatively strong bond, needs a lot of energy to be broken.
Angle of ~105º between H atoms means they are both on the “same side” of the oxygen atom.
Polarity: slide negative change on the oxygen side, and slight positive charge on the hydrogen side makes the water dipolar.
Water molecule structure: properties
Polarity of water molecules means they orient themselves relative to one another.
Molecules linked by a hydrogen bond.
Much weaker than covalent bonds, but strong enough to make molecules stick together.
Water is cohesive.
Also give water surface tension.
Properties of water: universal solvent
Water molecules not only stick to one another, but. to other polar chemical compounds.
Can reduce the attraction between ions of opposite charges by as much as 80%
For example: when putting table salt in water
The negative chloride will align with the positive side of the water molecule.
The positive sodium will align with the negative side of the water molecule.
The bonds of the salt ion become weakened and then dissolved into water.
Heat vs. temperature:
Heat: the transfer of thermal energy from a body with a hotter temperature to one with a cooler temperature.
The movement of energy.
Temperature: a measure of the average kinetic energy of particles.
Measure of energy within a substance
Heat capacity:
Amount of energy required to raise the temperature of a substance by 1 degreed centigrade.
High heat capacity leads to high thermal inertia: more heat is necessary to change the temperature.
Low heat capacity means temperature will change faster with same amount of energy added.
Water has a high heat capacity so temperature changes in the ocean are relatively small.
Specific heat (capacity):
Heat capacity per unit mass (heat needed to raise 1ºC per 1g).
Specific heat (capacity): importance
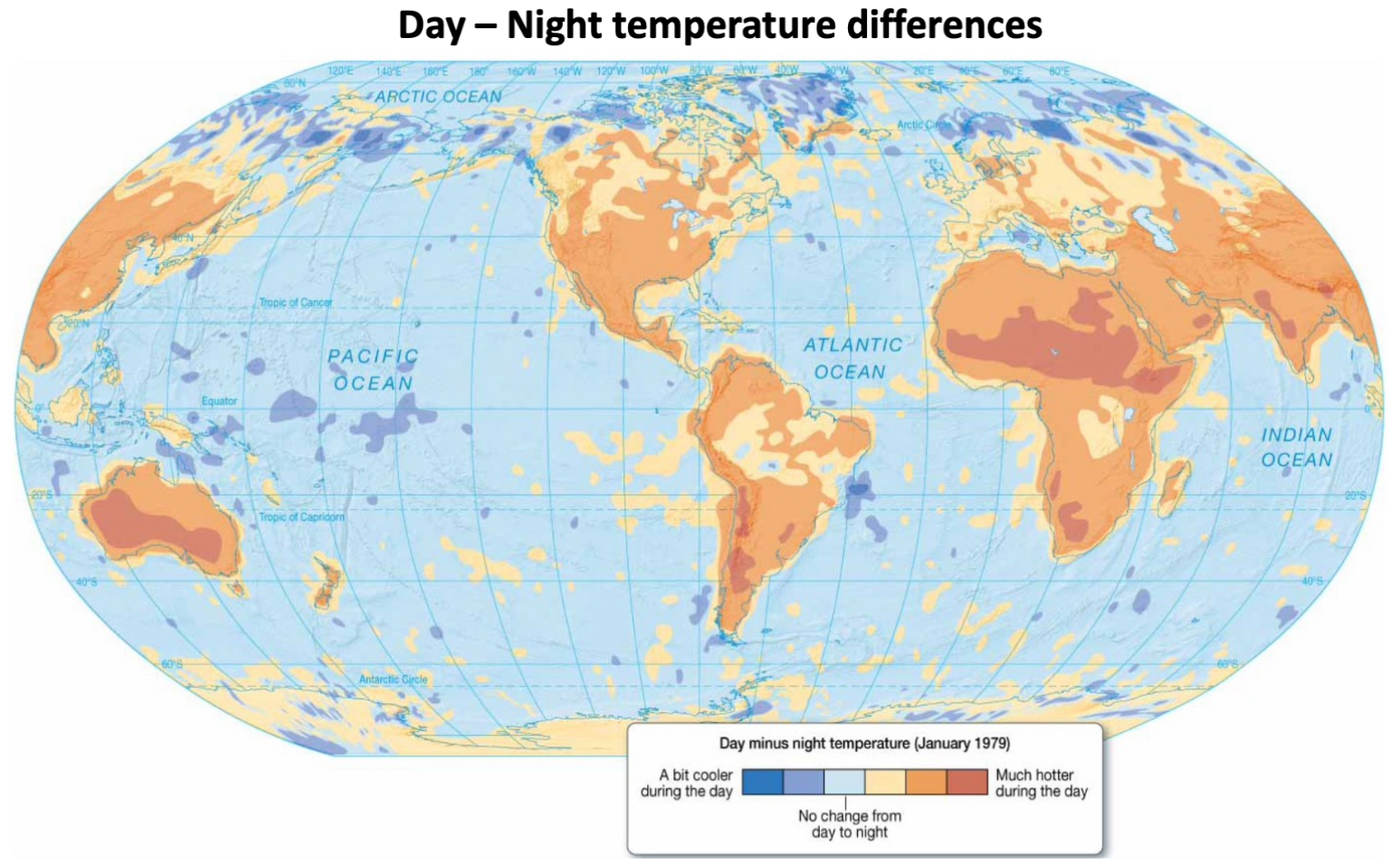
The ocean holds a pretty constant temperature, its temperature does not fluctuate much is response to the changing seasons (compared to land/rock which has a lower eat capacity and therefore changes in temperature happen more quickly.
As you go closer inland, and away from the ocean, the changes are more extreme and it is much hotter during the day.
Ocean is colder during the day in the Arctic because the sunlight causes ice to melt into the ocean which makes the water colder.
Deserts have the largest differences in temperature between day and night.
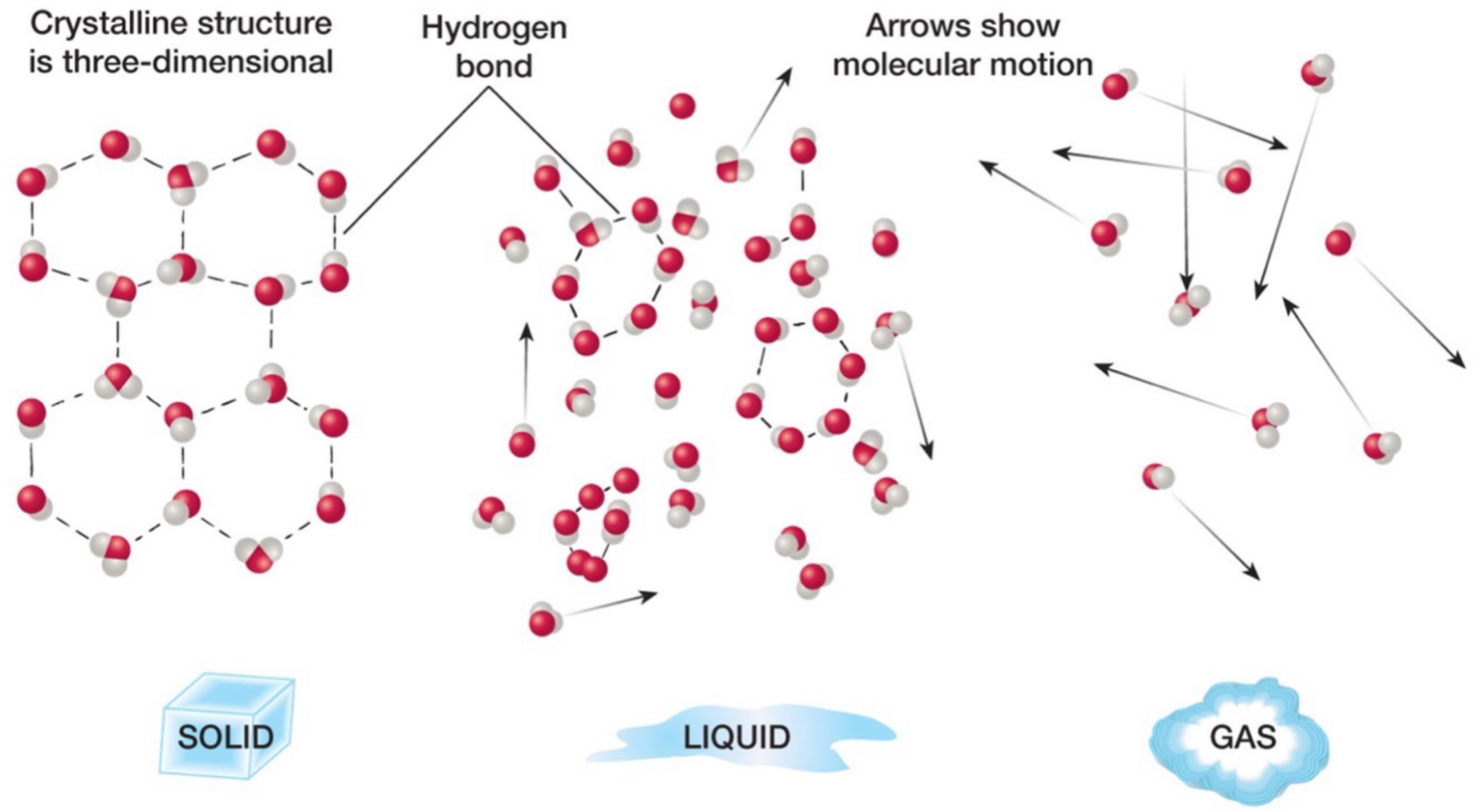
Phases of water: solid
H bonds between all molecules.
Molecules are aligned in a certain way, fixed and rigid structure.
Molecules not moving a lot.
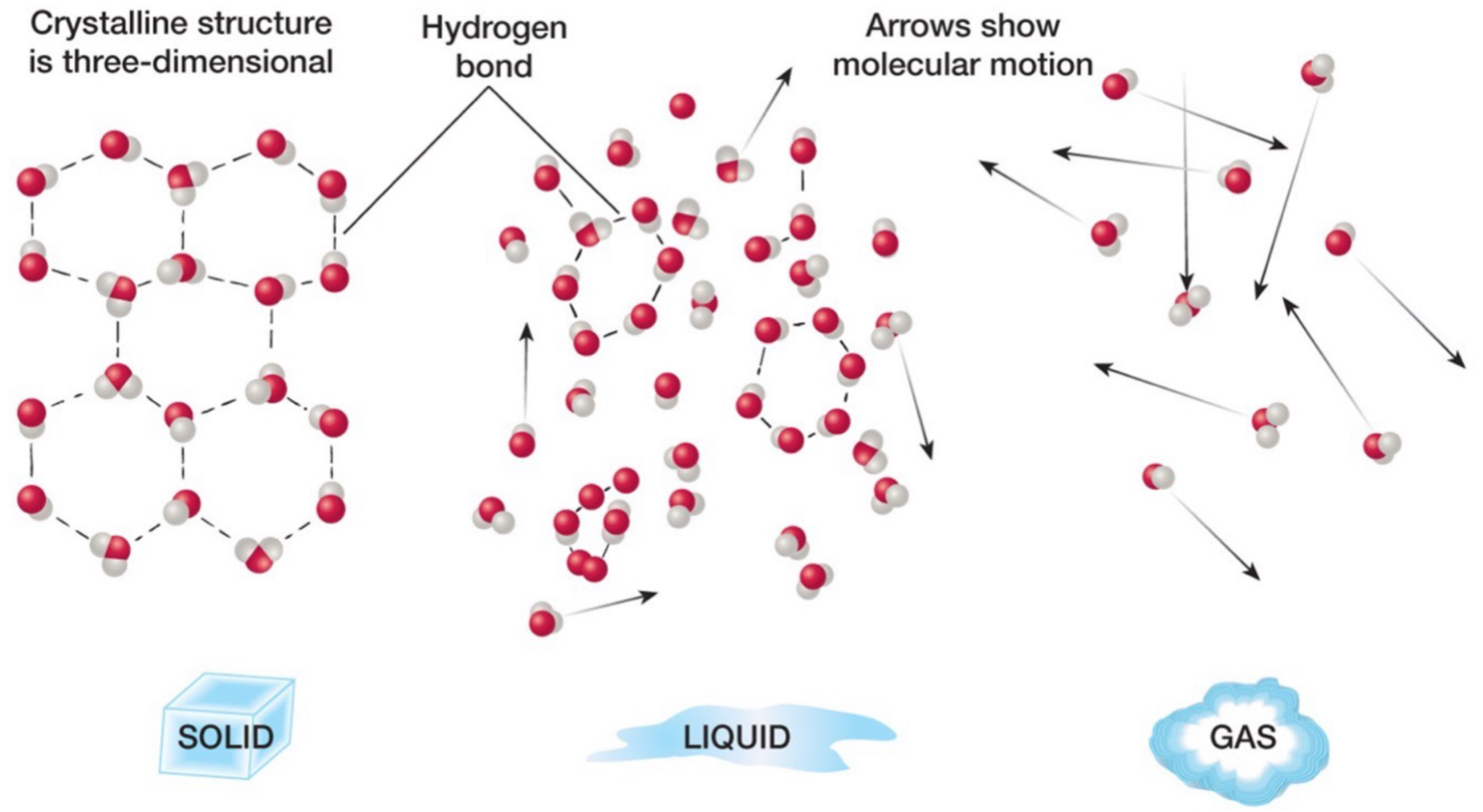
Phases of water: liquid
H bonds between some molecules.
Molecules moving a medium amount.
Most of the water we have is in liquid form.
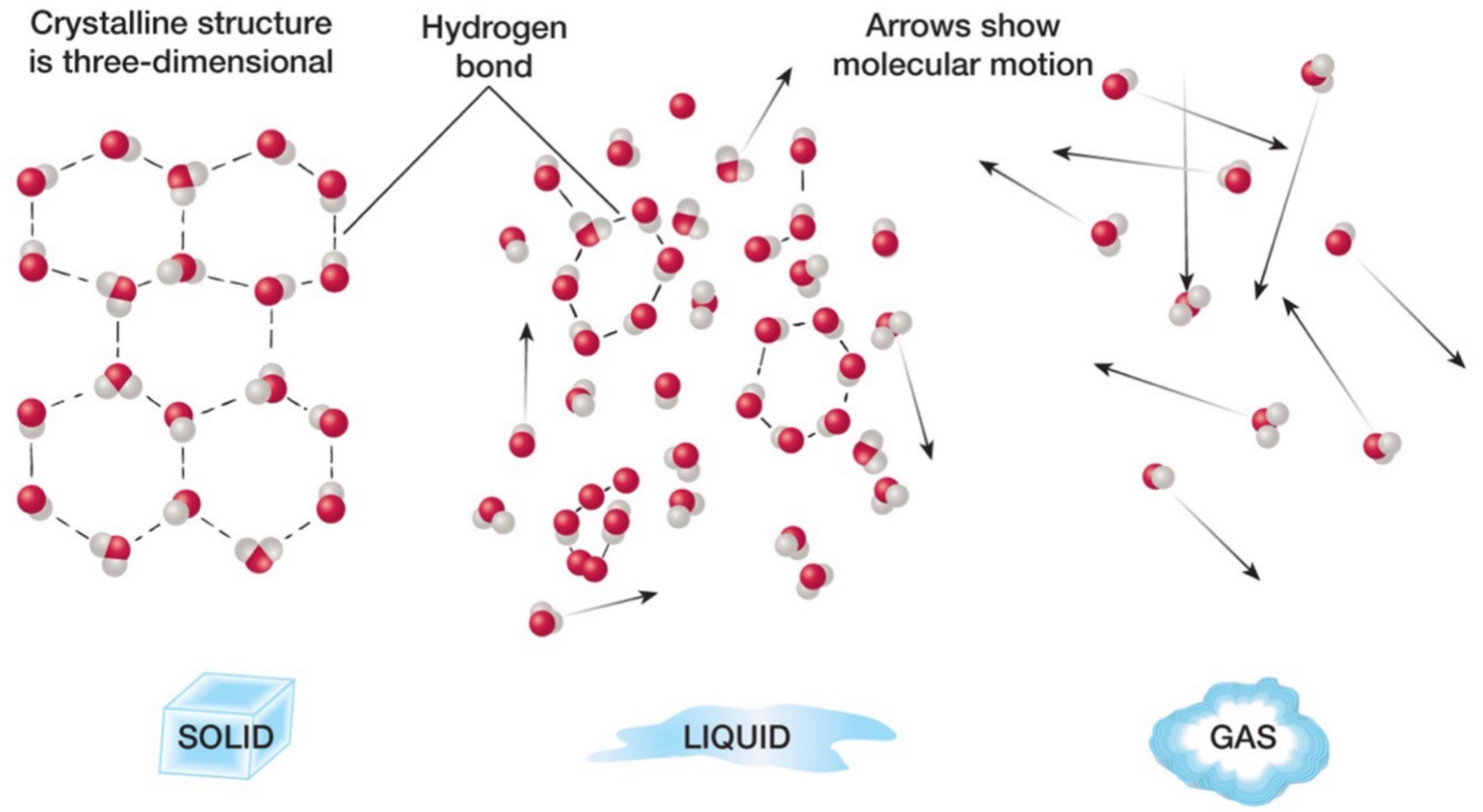
Phases of water: gas
No H bonds.
A bunch of individual molecules.
Molecules are moving everywhere.
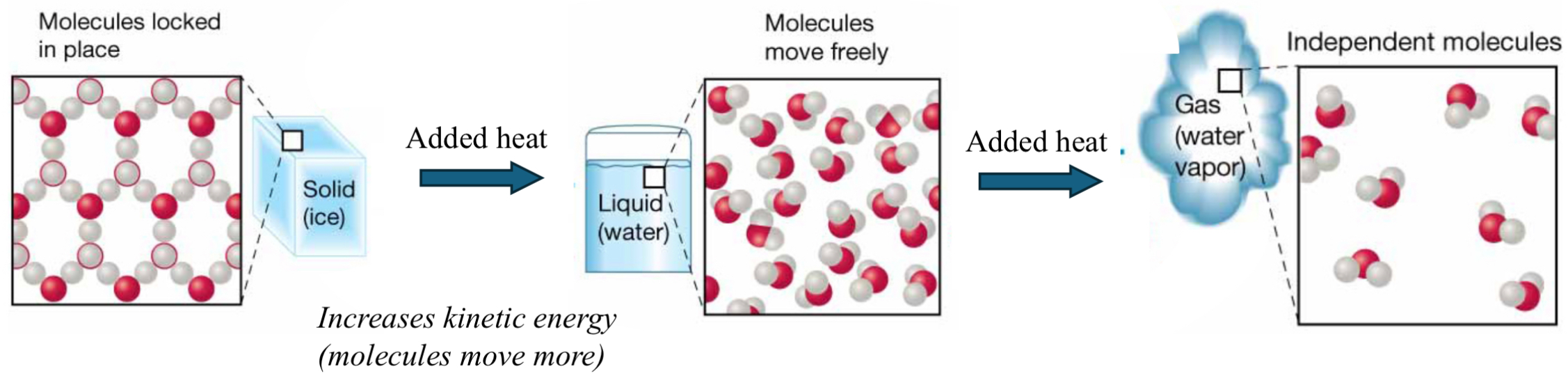
Phases of water: transition
Change phases (solid → water → gas) requires adding heat (adding energy).
Thus, increasing kinetic energy which means molecules will want to more more freely and will start breaking bonds apart from each other.
Phase transition: solid → water
Melting.
(+) absorbs heat from its environment.
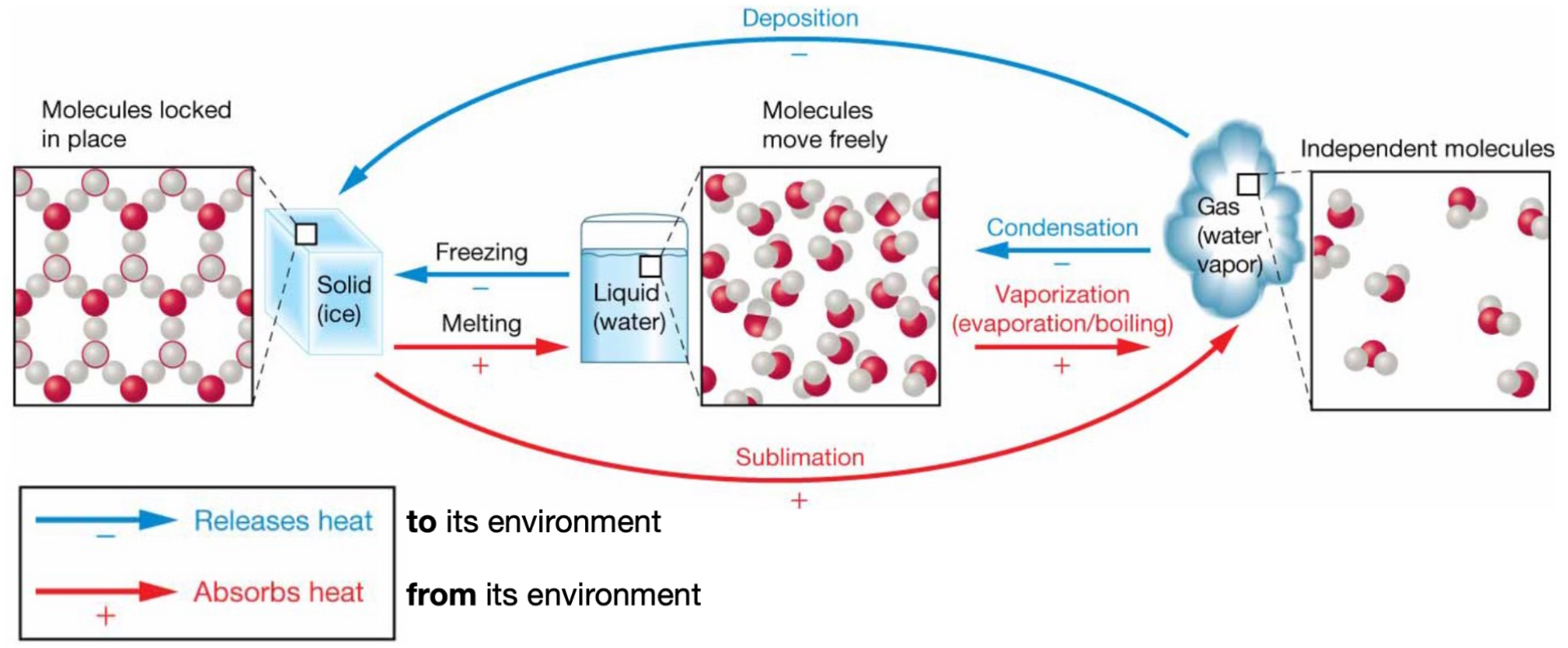
Phase transition: water → gas
Vaporization (evaporation/boiling).
(+) absorbs heat from its environment.
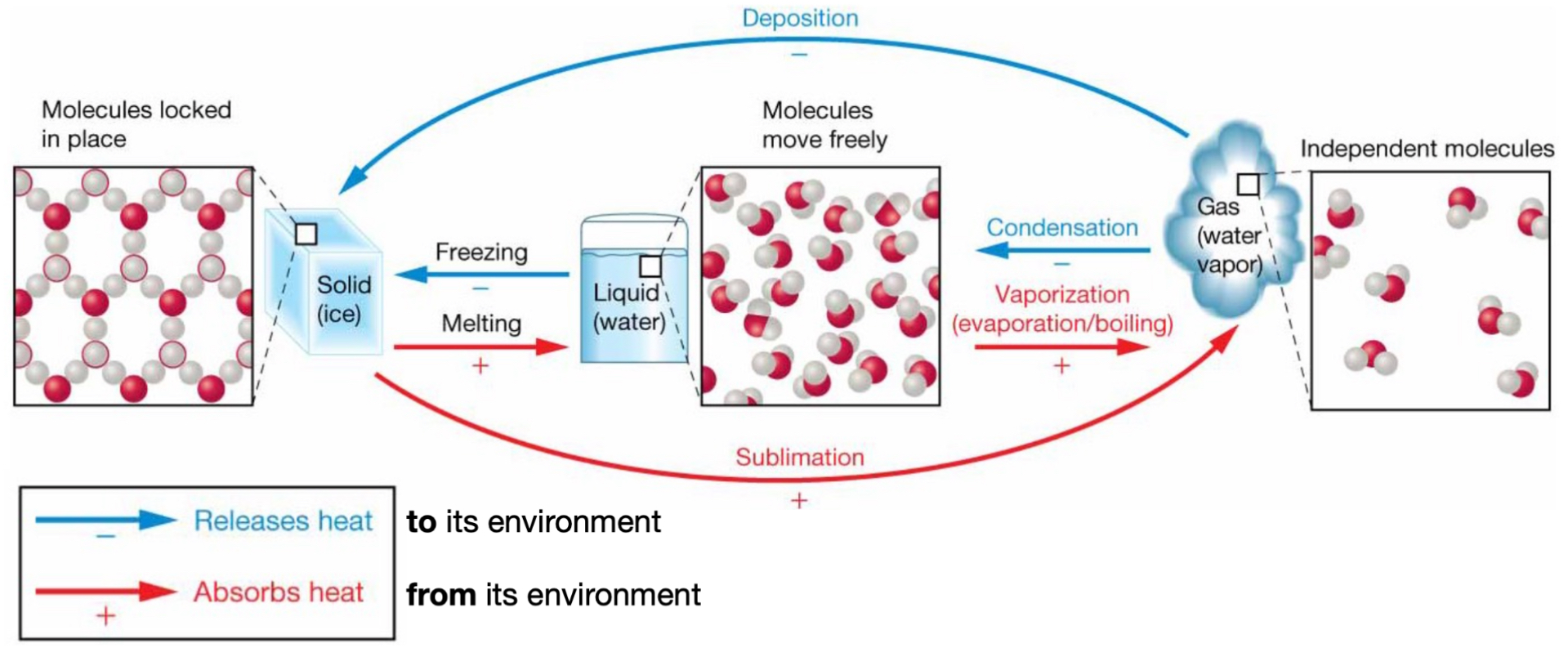
Phase transition: gas → water
Condensation.
(-) releases heat to its environment.
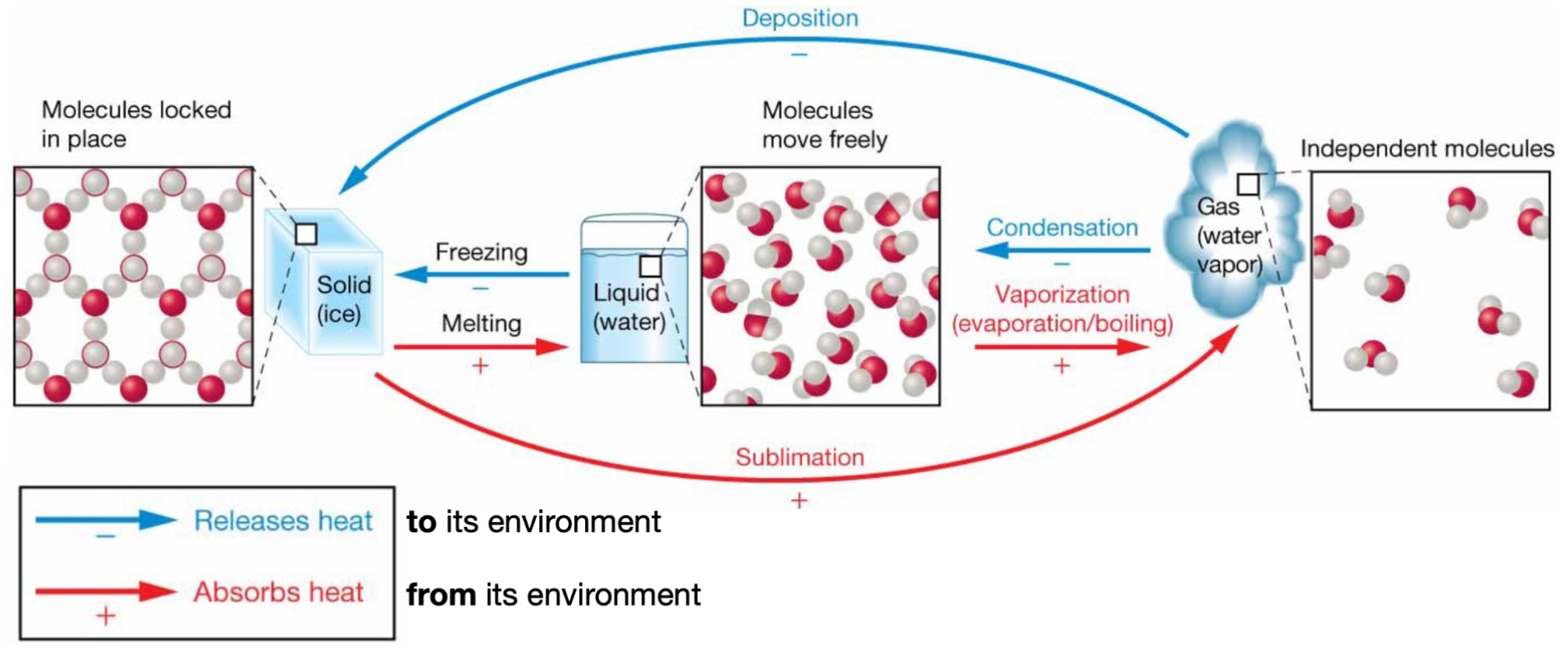
Phase transition: water → solid
Freezing.
(-) releases heat to its environment.
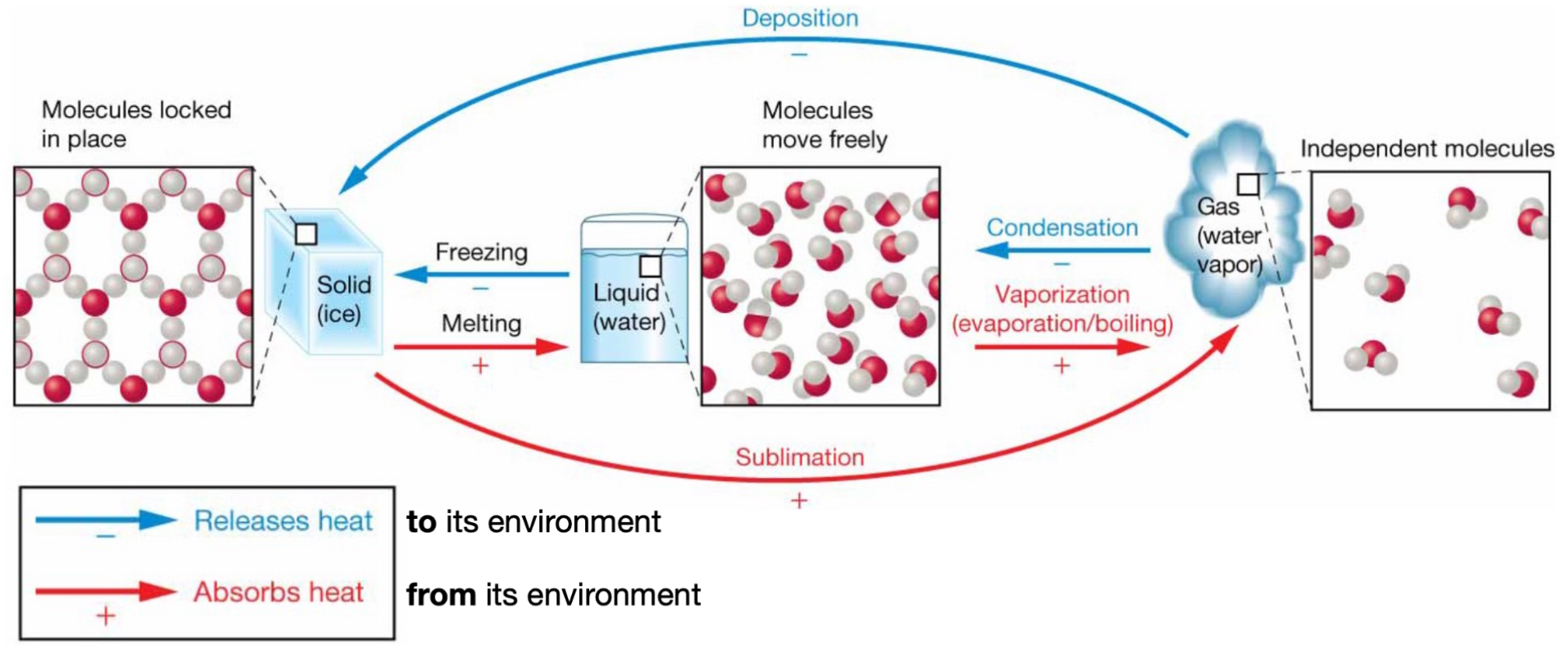
Phase transition: solid → gas
Sublimation.
(+) absorbs heat from its environment.
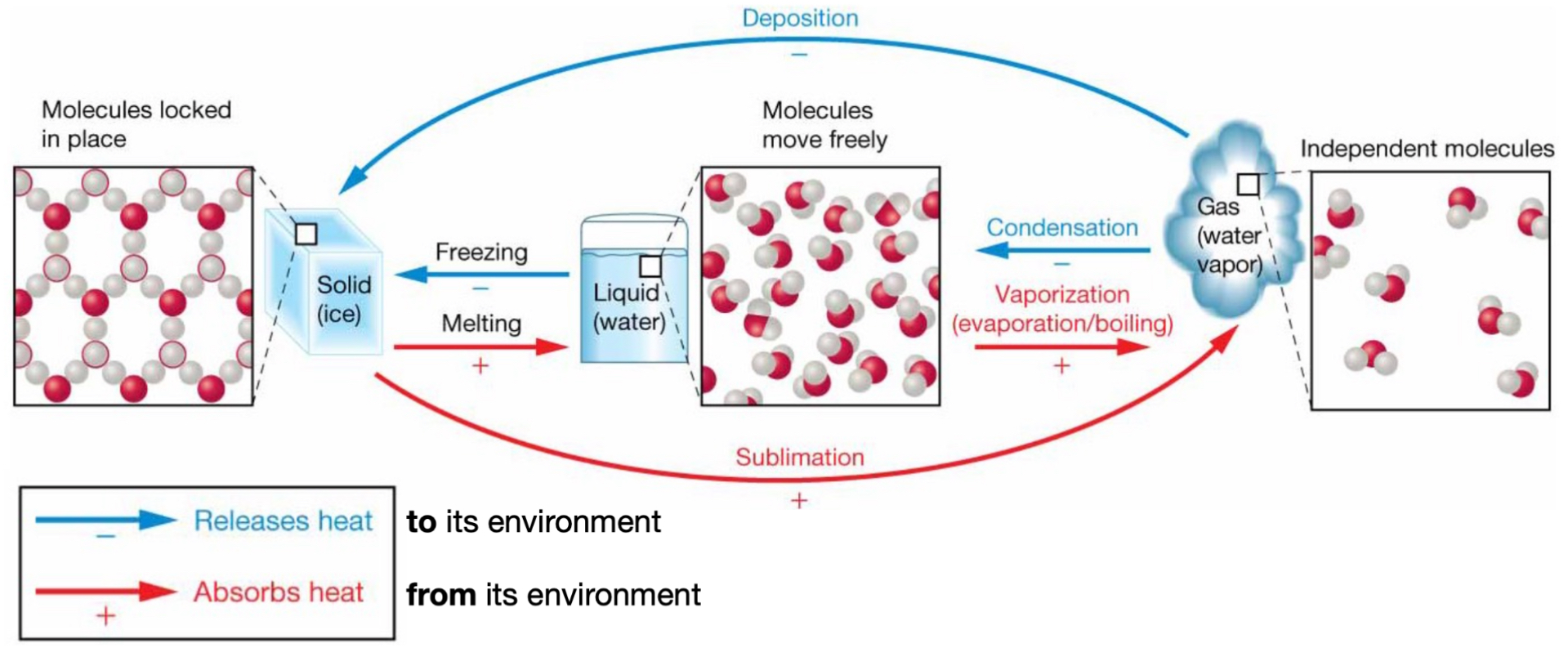
Phase transition: gas → solid
Deposition.
(-) releases heat to the environment.
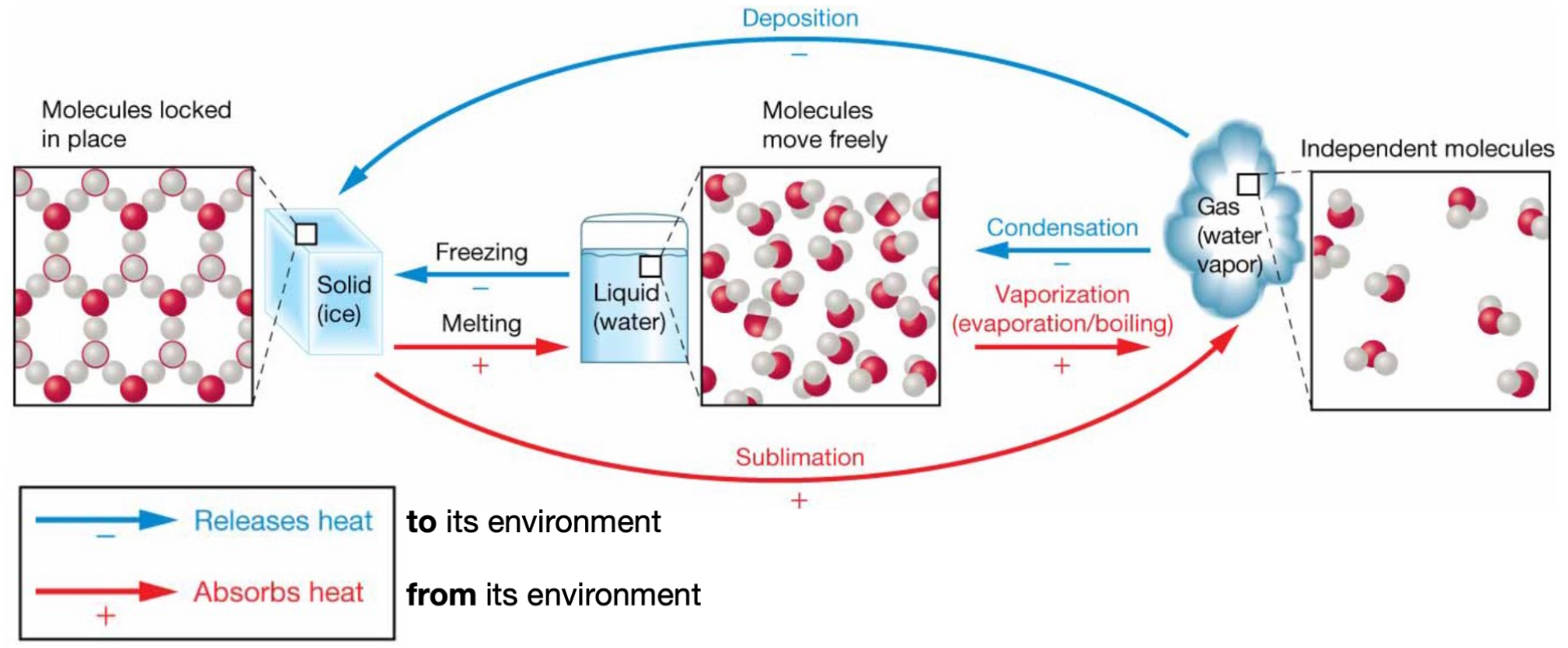
Latent heat:
Energy is added, but temperature does not change.
“Hidden away” to drive phase change.
Large latent heat of vaporization to break hydrogen bonds - all H bonds need to be broken versus just some for transition from solid to liquid.
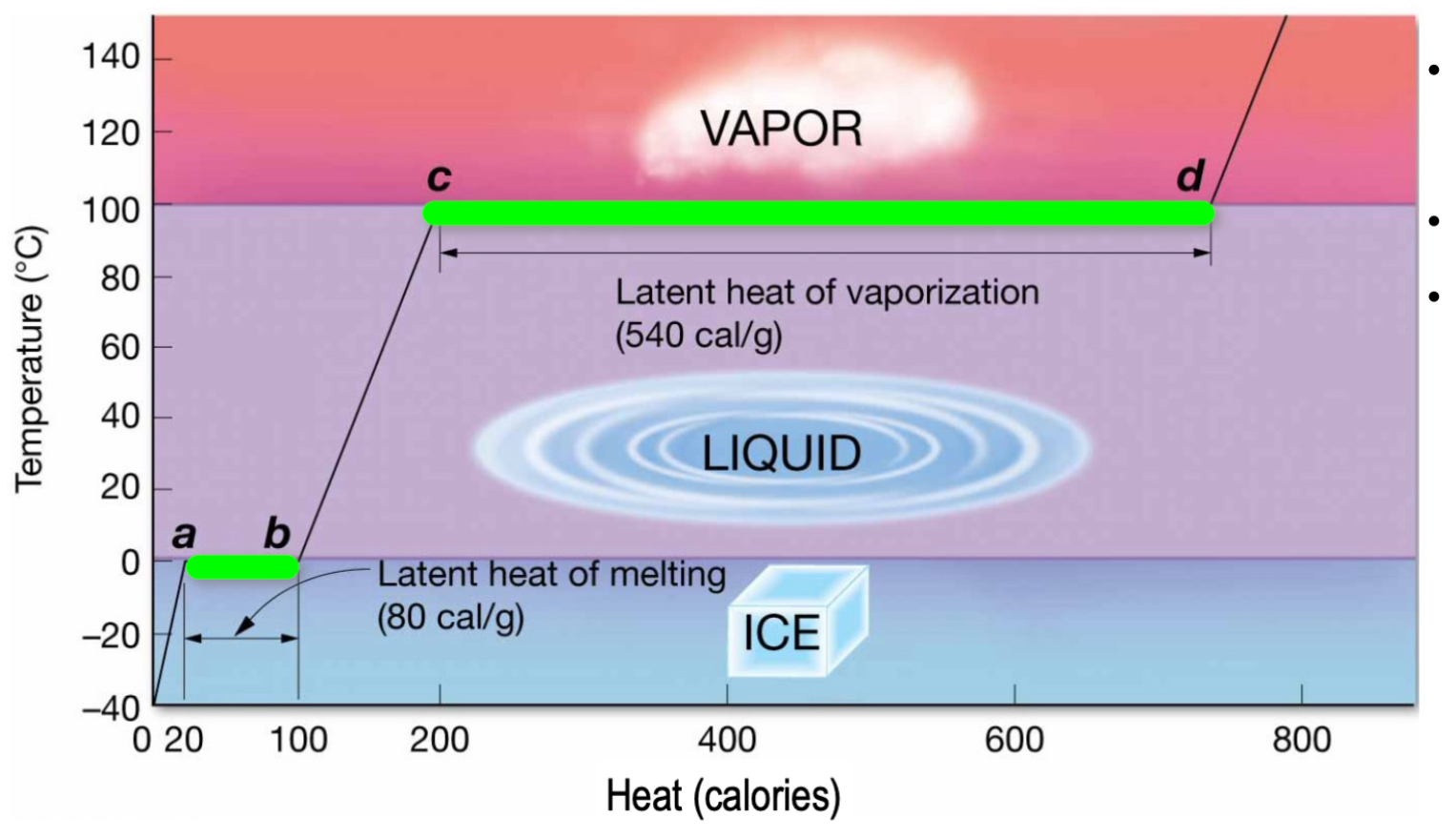
Evaporation vs. boiling:
Unlike boiling, evaporation can happen at lower temperatures.
In higher temperatures, molecules will vibrate more (and move from liquid to gas phase).
Molecules are constantly trying to reach an equilibrium.
Latent heat: example
In the same place, one day is sunny and one day is snowing:
The sunny day will heat up more than the snowy day since some of the heat will be allocated to melting snow.

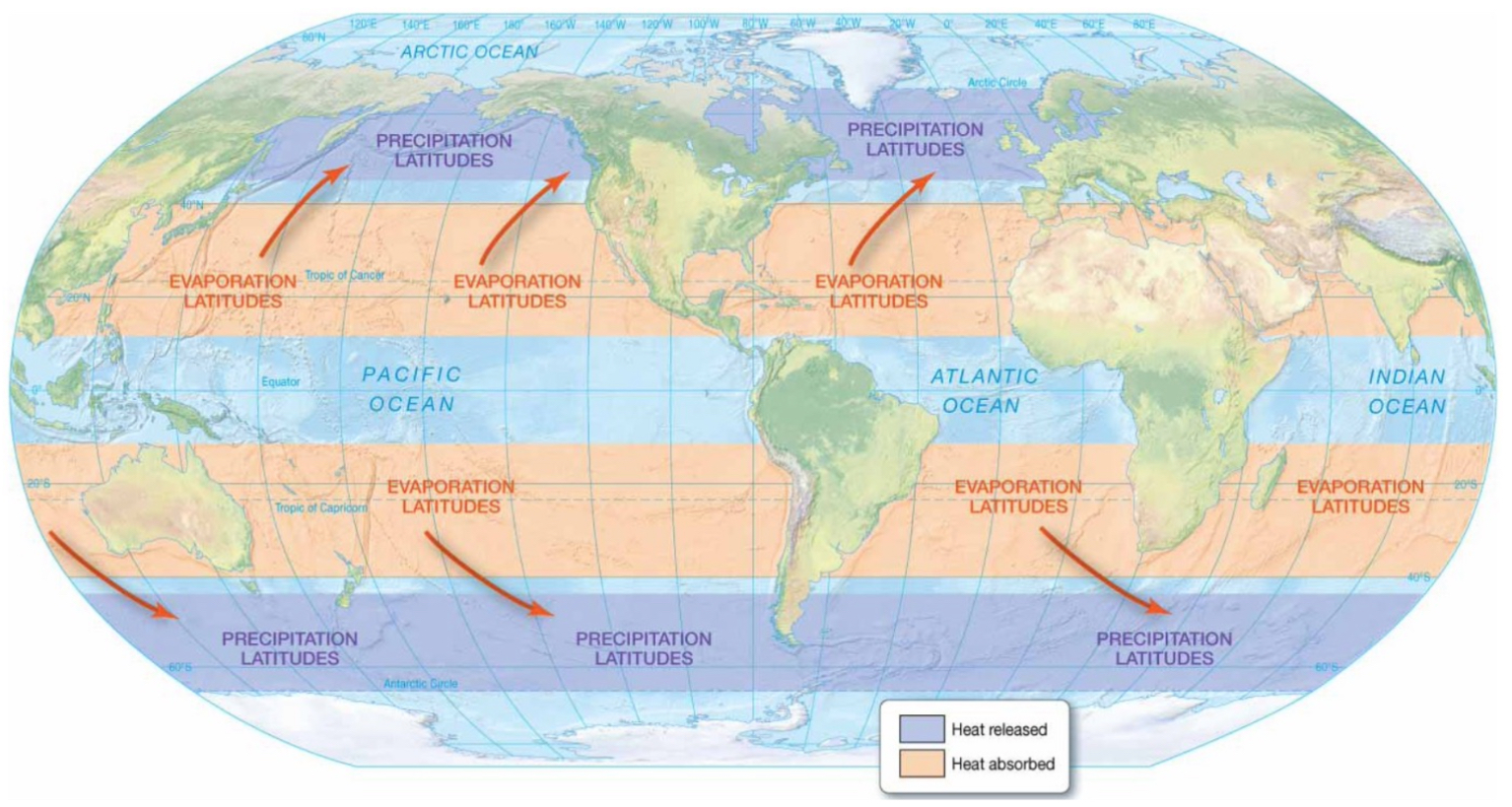
Global effects of latent heat:
Evaporation and precipitation can transfer heat vertically.
Evaporation: absorbs energy from the environment.
Precipitation: releases energy.
Globally, it helps redistribute the energy that is absorbed from the sun by moving energy from low latitudes to high latitudes.
Freshwater vs. saltwater:
Salinity: total amount of dissolve inorganic solids in the water.
Ions enter the ocean through these processes:
River discharge.
Volcanic eruptions.
Hydrothermal activity at the mid-ocean ridge.
Ocean is salty because rocks and sediment on the are eroding into the ocean.
Ions are removed from the ocean through these properties:
Adsorption and precipitation.
Sea spray.
Biological processes.
Hydrothermal activity at the mid-ocean ridge
Properties of water: relatively high boiling and freezing points
Change in phase leads to change in density which comes from volume, not mass.
For example, 1g of water takes up 9% less space than 1g ice.
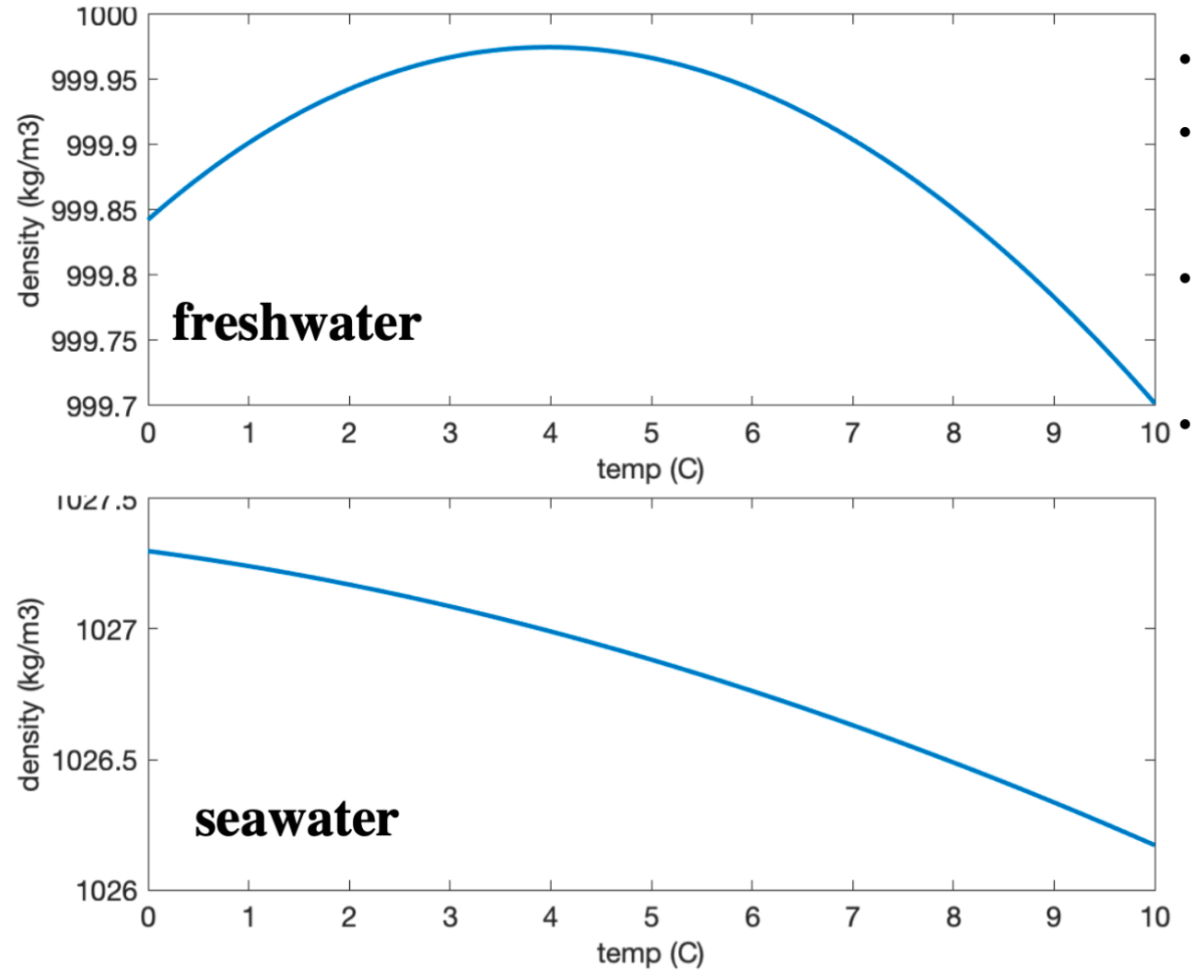
Freshwater vs. saltwater: temperature effects
Warmer water is less dense.
Seawater density is also influenced by the salt it contains.
There are nonlinear effects of salt and temperature on water density.
Seawater does not peak in density before freezing.
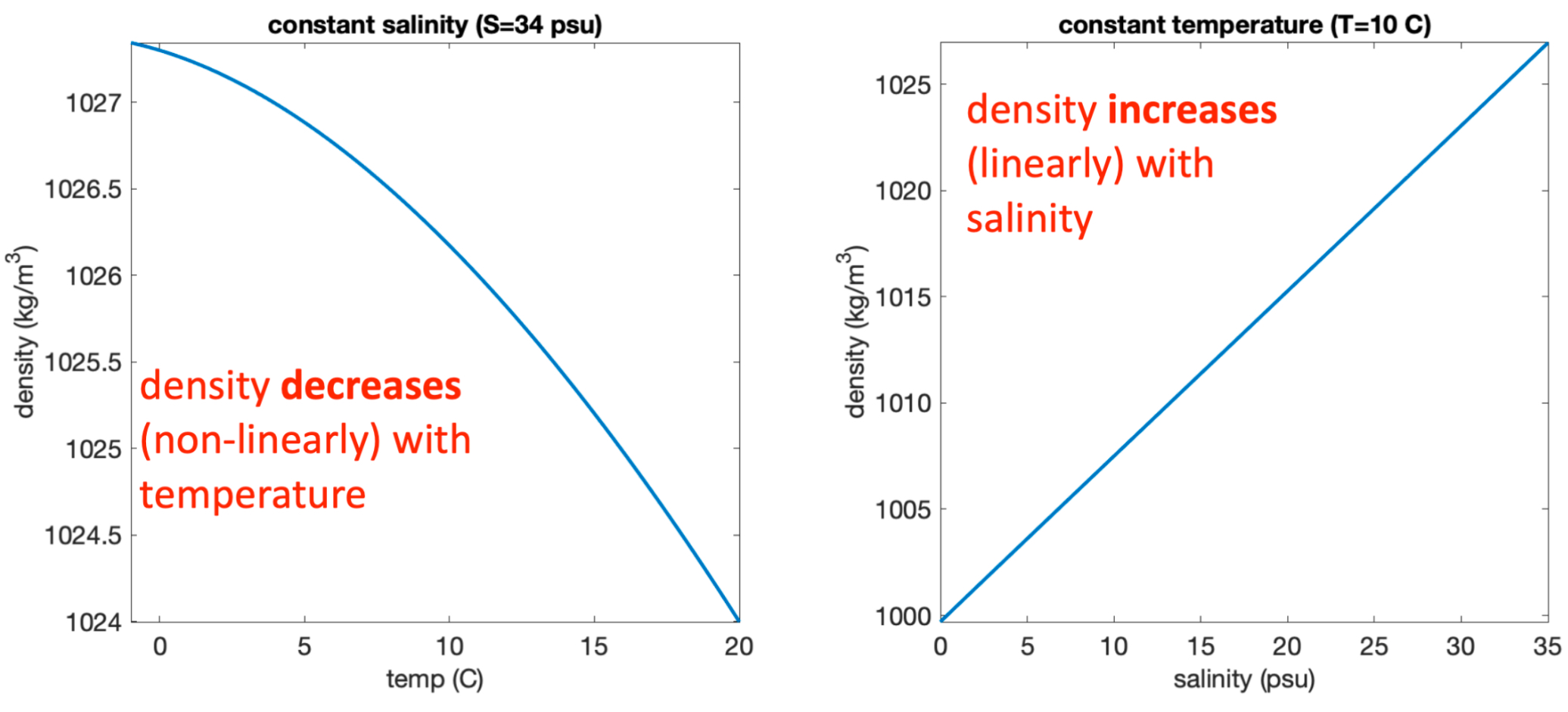
Salinity & temperature effects on density:
Density decreases (non-linearly) with temperature.
Density increases (linearly) with salinity.
Density differences will cause stratification:
This can be found between different mediums (water, oil, dish soap, etc).
Also true for different parcels of water of different temperature and salinity.
Properties of water: relatively low viscosity
Viscosity: the internal friction of a liquid.
Low viscosity: weak intermolecular bonds (i.e. water).
Medium viscosity: medium strength intermolecular bonds (i.e. olive oil).
High viscosity: strong intermolecular bonds (i.e. honey).